IMPACT OF THE METHYLENETETRAHYDROFOLATE REDUCTASE (MTHFR) C677T GENE POLYMORPHISM ON CLINICAL OUTCOMES OF HLA-MATCHED SIBLING ALLOGENEIC HEMATOPOIETIC STEM CELL TRANSPLANTATION
(Abstract release date: 05/19/16)
EHA Library. Mohammed Saleh M. 06/09/16; 135059; PB2159

Dr. Mostafa Mohammed Saleh
Contributions
Contributions
Abstract
Abstract: PB2159
Type: Publication Only
Background
Allogeneic hematopoietic stem cell transplantation (HSCT) is widely used to treat various hematological malignant and bengin diseases. The occurrence of complications following HSCT-as graft versus host disease (GVHD), hepatic veno-occlusive disease (VOD), oral mucositis (OM), drug induced hepatic and renal adverse events- is highly variable and dependent on a multitude of host, donor, and treatment factors. Identifying important genetic variables will allow for better prediction of HSCT-related outcomes, that could help to develop targeted interventions. A common genetic polymorphism, C677T, has been described for Methylenetetrahydrofolate reductase (MTHFR);results in amino acid changes, at codons 222. The homozygous 677TT genotype has been shown to have 30% of the MTHFR wild-type enzyme activity in vitro, and the heterozygous (CT) genotype has approximately 60% of wild-type enzyme activity (Liew and Gupta, 2015 Eur J Med Genet, 58, 1-10 ).
Aims
To evaluate impact of the C677T polymorphism of 5,10-methylenetetrahydrofolate reductase (MTHFR) on the clinical outcomes of patients treated using human leukocyte antigen-matched sibling stem cell transplantation as acute GVHD,VOD,severe oral mucositis (SOM),drug induced hepatic and renal toxicity, transplant related mortality(TRM) and overall survival(OS).
Methods
The study subjects were 46 patients receiving allogeneic HSCT at a bone marrow transplantation unit in Nasser institute for research and treatment from 2010 to 2014,with complete clinical records ; and DNA available for genotyping. MTHFR genotyping was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).Acute GVHD was assessed using conventional criteria (Jacobsohn and Vogelsang, 2007 Orphanet J Rare Dis, 2, 35), necessity of parenteral nutrition used to identify SOM , and VOD of the liver was defined according to McDonald et al.(1993 Ann Intern Med, 118, 255-67).We evaluated renal and hepatic toxicity based on peak serum creatinine and peak total bilirubin, AST, and ALT levels during the first 30 days after allogeneic HSCT.TRM was defined as death by any cause during the course of treatment, other than the relapse of underlying disease.
Results
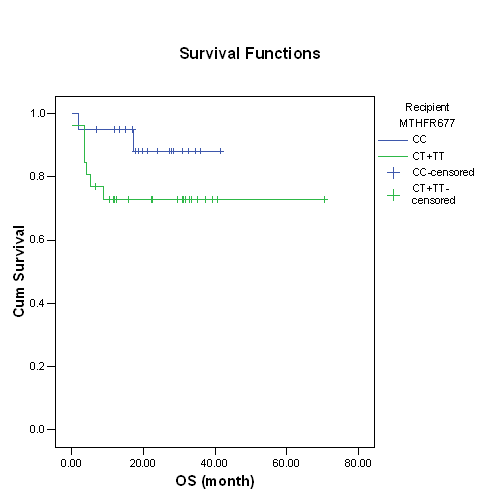
Median age at the time of HSCT was 22 years (range 3–42 years); 32 patients (69.6%) above > 18 years, and the median follow-up period of survivors was 21 months. Acute myeloid leukemia (AML) was the most common underlying diagnosis 23 patients (50%) of them 12 (26%) are in complete remission 1 (CR1).The most commonly used conditioning regimen consisted of Busulfan (BU), and Cyclophosphamide (CY) (n=26, 56.5%). All received peripheral blood stem as a stem cell source with mean CD34+ stem cell dose (6.6+2.3) 106/kg. Twenty-seven patients (58.7%) were males. Methotrexate (MTX) in addition to Cyclosporine (CsA) was used as GVHD prophylaxis in 40 patients (87%).The frequencies of the MTHFR C677T genotypes in patients were 43.5% (20 patients) for 677CC, 50% (23 patients) for 677CT, and 6.5% (3 patients) for 677TT ;the allelic frequency of the 677T was 31.5% . Recipient MTHFR677 in CT or TT showed higher incidence of acute GVHD (7/26) 26.9% versus (2/20) 10% in CC, but not statistically significant; p=0.26. MTHFR C677T in CT or TT showed higher incidence of hepatic toxicity (11/26) 42.3% versus (5/20) 25% in CC; also higher transplant related mortality (5/26) 19.2% versus (2/20) 10% in CC, but not statistically significant; p=0.22 & 0.45 respectively.In log rank survival analysis,recipients with variant allele MTHFR 677T were associated with lower non statistically significant overall survival; p=0.15.VOD was diagnosed in 1 patient (2.2%) and had heterogeneous status of the polymorphism, MTHFR 677 CT genotype.
Conclusion
Genotyping for MTHFR C677T before HSCT could have clinical significance, not statistically proven in our study, in prediction patients at high risk of developing poor outcomes. Large multicentric, highly standardized prospective studies are needed to identify such potential pharmacogenetic markers with sufficiently strong evidence to be used in clinical practice.
Session topic: E-poster
Keyword(s): Allogeneic hematopoietic stem cell transplant, Gene polymorphism, Methylene tetrahydrofolate reductase
Type: Publication Only
Background
Allogeneic hematopoietic stem cell transplantation (HSCT) is widely used to treat various hematological malignant and bengin diseases. The occurrence of complications following HSCT-as graft versus host disease (GVHD), hepatic veno-occlusive disease (VOD), oral mucositis (OM), drug induced hepatic and renal adverse events- is highly variable and dependent on a multitude of host, donor, and treatment factors. Identifying important genetic variables will allow for better prediction of HSCT-related outcomes, that could help to develop targeted interventions. A common genetic polymorphism, C677T, has been described for Methylenetetrahydrofolate reductase (MTHFR);results in amino acid changes, at codons 222. The homozygous 677TT genotype has been shown to have 30% of the MTHFR wild-type enzyme activity in vitro, and the heterozygous (CT) genotype has approximately 60% of wild-type enzyme activity (Liew and Gupta, 2015 Eur J Med Genet, 58, 1-10 ).
Aims
To evaluate impact of the C677T polymorphism of 5,10-methylenetetrahydrofolate reductase (MTHFR) on the clinical outcomes of patients treated using human leukocyte antigen-matched sibling stem cell transplantation as acute GVHD,VOD,severe oral mucositis (SOM),drug induced hepatic and renal toxicity, transplant related mortality(TRM) and overall survival(OS).
Methods
The study subjects were 46 patients receiving allogeneic HSCT at a bone marrow transplantation unit in Nasser institute for research and treatment from 2010 to 2014,with complete clinical records ; and DNA available for genotyping. MTHFR genotyping was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).Acute GVHD was assessed using conventional criteria (Jacobsohn and Vogelsang, 2007 Orphanet J Rare Dis, 2, 35), necessity of parenteral nutrition used to identify SOM , and VOD of the liver was defined according to McDonald et al.(1993 Ann Intern Med, 118, 255-67).We evaluated renal and hepatic toxicity based on peak serum creatinine and peak total bilirubin, AST, and ALT levels during the first 30 days after allogeneic HSCT.TRM was defined as death by any cause during the course of treatment, other than the relapse of underlying disease.
Results
Median age at the time of HSCT was 22 years (range 3–42 years); 32 patients (69.6%) above > 18 years, and the median follow-up period of survivors was 21 months. Acute myeloid leukemia (AML) was the most common underlying diagnosis 23 patients (50%) of them 12 (26%) are in complete remission 1 (CR1).The most commonly used conditioning regimen consisted of Busulfan (BU), and Cyclophosphamide (CY) (n=26, 56.5%). All received peripheral blood stem as a stem cell source with mean CD34+ stem cell dose (6.6+2.3) 106/kg. Twenty-seven patients (58.7%) were males. Methotrexate (MTX) in addition to Cyclosporine (CsA) was used as GVHD prophylaxis in 40 patients (87%).The frequencies of the MTHFR C677T genotypes in patients were 43.5% (20 patients) for 677CC, 50% (23 patients) for 677CT, and 6.5% (3 patients) for 677TT ;the allelic frequency of the 677T was 31.5% . Recipient MTHFR677 in CT or TT showed higher incidence of acute GVHD (7/26) 26.9% versus (2/20) 10% in CC, but not statistically significant; p=0.26. MTHFR C677T in CT or TT showed higher incidence of hepatic toxicity (11/26) 42.3% versus (5/20) 25% in CC; also higher transplant related mortality (5/26) 19.2% versus (2/20) 10% in CC, but not statistically significant; p=0.22 & 0.45 respectively.In log rank survival analysis,recipients with variant allele MTHFR 677T were associated with lower non statistically significant overall survival; p=0.15.VOD was diagnosed in 1 patient (2.2%) and had heterogeneous status of the polymorphism, MTHFR 677 CT genotype.
Conclusion
Genotyping for MTHFR C677T before HSCT could have clinical significance, not statistically proven in our study, in prediction patients at high risk of developing poor outcomes. Large multicentric, highly standardized prospective studies are needed to identify such potential pharmacogenetic markers with sufficiently strong evidence to be used in clinical practice.

Session topic: E-poster
Keyword(s): Allogeneic hematopoietic stem cell transplant, Gene polymorphism, Methylene tetrahydrofolate reductase
Abstract: PB2159
Type: Publication Only
Background
Allogeneic hematopoietic stem cell transplantation (HSCT) is widely used to treat various hematological malignant and bengin diseases. The occurrence of complications following HSCT-as graft versus host disease (GVHD), hepatic veno-occlusive disease (VOD), oral mucositis (OM), drug induced hepatic and renal adverse events- is highly variable and dependent on a multitude of host, donor, and treatment factors. Identifying important genetic variables will allow for better prediction of HSCT-related outcomes, that could help to develop targeted interventions. A common genetic polymorphism, C677T, has been described for Methylenetetrahydrofolate reductase (MTHFR);results in amino acid changes, at codons 222. The homozygous 677TT genotype has been shown to have 30% of the MTHFR wild-type enzyme activity in vitro, and the heterozygous (CT) genotype has approximately 60% of wild-type enzyme activity (Liew and Gupta, 2015 Eur J Med Genet, 58, 1-10 ).
Aims
To evaluate impact of the C677T polymorphism of 5,10-methylenetetrahydrofolate reductase (MTHFR) on the clinical outcomes of patients treated using human leukocyte antigen-matched sibling stem cell transplantation as acute GVHD,VOD,severe oral mucositis (SOM),drug induced hepatic and renal toxicity, transplant related mortality(TRM) and overall survival(OS).
Methods
The study subjects were 46 patients receiving allogeneic HSCT at a bone marrow transplantation unit in Nasser institute for research and treatment from 2010 to 2014,with complete clinical records ; and DNA available for genotyping. MTHFR genotyping was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).Acute GVHD was assessed using conventional criteria (Jacobsohn and Vogelsang, 2007 Orphanet J Rare Dis, 2, 35), necessity of parenteral nutrition used to identify SOM , and VOD of the liver was defined according to McDonald et al.(1993 Ann Intern Med, 118, 255-67).We evaluated renal and hepatic toxicity based on peak serum creatinine and peak total bilirubin, AST, and ALT levels during the first 30 days after allogeneic HSCT.TRM was defined as death by any cause during the course of treatment, other than the relapse of underlying disease.
Results
Median age at the time of HSCT was 22 years (range 3–42 years); 32 patients (69.6%) above > 18 years, and the median follow-up period of survivors was 21 months. Acute myeloid leukemia (AML) was the most common underlying diagnosis 23 patients (50%) of them 12 (26%) are in complete remission 1 (CR1).The most commonly used conditioning regimen consisted of Busulfan (BU), and Cyclophosphamide (CY) (n=26, 56.5%). All received peripheral blood stem as a stem cell source with mean CD34+ stem cell dose (6.6+2.3) 106/kg. Twenty-seven patients (58.7%) were males. Methotrexate (MTX) in addition to Cyclosporine (CsA) was used as GVHD prophylaxis in 40 patients (87%).The frequencies of the MTHFR C677T genotypes in patients were 43.5% (20 patients) for 677CC, 50% (23 patients) for 677CT, and 6.5% (3 patients) for 677TT ;the allelic frequency of the 677T was 31.5% . Recipient MTHFR677 in CT or TT showed higher incidence of acute GVHD (7/26) 26.9% versus (2/20) 10% in CC, but not statistically significant; p=0.26. MTHFR C677T in CT or TT showed higher incidence of hepatic toxicity (11/26) 42.3% versus (5/20) 25% in CC; also higher transplant related mortality (5/26) 19.2% versus (2/20) 10% in CC, but not statistically significant; p=0.22 & 0.45 respectively.In log rank survival analysis,recipients with variant allele MTHFR 677T were associated with lower non statistically significant overall survival; p=0.15.VOD was diagnosed in 1 patient (2.2%) and had heterogeneous status of the polymorphism, MTHFR 677 CT genotype.
Conclusion
Genotyping for MTHFR C677T before HSCT could have clinical significance, not statistically proven in our study, in prediction patients at high risk of developing poor outcomes. Large multicentric, highly standardized prospective studies are needed to identify such potential pharmacogenetic markers with sufficiently strong evidence to be used in clinical practice.

Session topic: E-poster
Keyword(s): Allogeneic hematopoietic stem cell transplant, Gene polymorphism, Methylene tetrahydrofolate reductase
Type: Publication Only
Background
Allogeneic hematopoietic stem cell transplantation (HSCT) is widely used to treat various hematological malignant and bengin diseases. The occurrence of complications following HSCT-as graft versus host disease (GVHD), hepatic veno-occlusive disease (VOD), oral mucositis (OM), drug induced hepatic and renal adverse events- is highly variable and dependent on a multitude of host, donor, and treatment factors. Identifying important genetic variables will allow for better prediction of HSCT-related outcomes, that could help to develop targeted interventions. A common genetic polymorphism, C677T, has been described for Methylenetetrahydrofolate reductase (MTHFR);results in amino acid changes, at codons 222. The homozygous 677TT genotype has been shown to have 30% of the MTHFR wild-type enzyme activity in vitro, and the heterozygous (CT) genotype has approximately 60% of wild-type enzyme activity (Liew and Gupta, 2015 Eur J Med Genet, 58, 1-10 ).
Aims
To evaluate impact of the C677T polymorphism of 5,10-methylenetetrahydrofolate reductase (MTHFR) on the clinical outcomes of patients treated using human leukocyte antigen-matched sibling stem cell transplantation as acute GVHD,VOD,severe oral mucositis (SOM),drug induced hepatic and renal toxicity, transplant related mortality(TRM) and overall survival(OS).
Methods
The study subjects were 46 patients receiving allogeneic HSCT at a bone marrow transplantation unit in Nasser institute for research and treatment from 2010 to 2014,with complete clinical records ; and DNA available for genotyping. MTHFR genotyping was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).Acute GVHD was assessed using conventional criteria (Jacobsohn and Vogelsang, 2007 Orphanet J Rare Dis, 2, 35), necessity of parenteral nutrition used to identify SOM , and VOD of the liver was defined according to McDonald et al.(1993 Ann Intern Med, 118, 255-67).We evaluated renal and hepatic toxicity based on peak serum creatinine and peak total bilirubin, AST, and ALT levels during the first 30 days after allogeneic HSCT.TRM was defined as death by any cause during the course of treatment, other than the relapse of underlying disease.
Results
Median age at the time of HSCT was 22 years (range 3–42 years); 32 patients (69.6%) above > 18 years, and the median follow-up period of survivors was 21 months. Acute myeloid leukemia (AML) was the most common underlying diagnosis 23 patients (50%) of them 12 (26%) are in complete remission 1 (CR1).The most commonly used conditioning regimen consisted of Busulfan (BU), and Cyclophosphamide (CY) (n=26, 56.5%). All received peripheral blood stem as a stem cell source with mean CD34+ stem cell dose (6.6+2.3) 106/kg. Twenty-seven patients (58.7%) were males. Methotrexate (MTX) in addition to Cyclosporine (CsA) was used as GVHD prophylaxis in 40 patients (87%).The frequencies of the MTHFR C677T genotypes in patients were 43.5% (20 patients) for 677CC, 50% (23 patients) for 677CT, and 6.5% (3 patients) for 677TT ;the allelic frequency of the 677T was 31.5% . Recipient MTHFR677 in CT or TT showed higher incidence of acute GVHD (7/26) 26.9% versus (2/20) 10% in CC, but not statistically significant; p=0.26. MTHFR C677T in CT or TT showed higher incidence of hepatic toxicity (11/26) 42.3% versus (5/20) 25% in CC; also higher transplant related mortality (5/26) 19.2% versus (2/20) 10% in CC, but not statistically significant; p=0.22 & 0.45 respectively.In log rank survival analysis,recipients with variant allele MTHFR 677T were associated with lower non statistically significant overall survival; p=0.15.VOD was diagnosed in 1 patient (2.2%) and had heterogeneous status of the polymorphism, MTHFR 677 CT genotype.
Conclusion
Genotyping for MTHFR C677T before HSCT could have clinical significance, not statistically proven in our study, in prediction patients at high risk of developing poor outcomes. Large multicentric, highly standardized prospective studies are needed to identify such potential pharmacogenetic markers with sufficiently strong evidence to be used in clinical practice.

Session topic: E-poster
Keyword(s): Allogeneic hematopoietic stem cell transplant, Gene polymorphism, Methylene tetrahydrofolate reductase
{{ help_message }}
{{filter}}


