OUTCOMES OF HIGH GRADE GASTROINTESTINAL GVHD POST-HSCT IN CHILDREN
(Abstract release date: 05/19/16)
EHA Library. Yeşilipek A. 06/09/16; 135056; PB2156

Dr. Akif Yeşilipek
Contributions
Contributions
Abstract
Abstract: PB2156
Type: Publication Only
Background
The high grade acute graft versus host disease (aGVHD) of typically involved organs (skin, gastrointestinal system (GIS) and liver) have specific features of therapeutic challenges, among which high grade GIS aGVHD has a distinctive and remarkable place in this context.
Aims
Our study intend to bring out the risk factors and clinical course of high grade gastrointestinal system (GIS) GVHD in children.
Methods
This is a retrospective analysis of 28 pediatric patients presented with a clinical diagnosis of stage 3 and 4 acute GVHD of the GIS who were selected from allogeneic hematopoetic stem cell transplantation (HSCT) performed. The demographics, the regimen used for conditioning and GVHD prophylaxis, clinical characteristics of GVHD including follow-up, laboratory parameters during GVHD, treatment modalities used for GVHD, response assessment in every week, complications of GVHD, and survival data were recorded.
Results
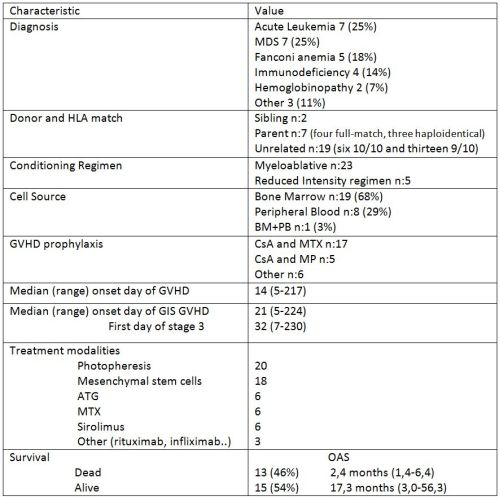
Patient and transplant characteristics were summarized in Table 1.
Conclusion
Overall survival at 3 months after the onset of stage 3 or 4 gut GVHD was 54%. Better outcome than the adult data might be related with more usage of bone marrow as a stem cell source or different characteristics of pediatric gastrointestinal system. In general, GVHD started 14 days post-HSCT, extended to GIS GVHD in a week and progressed to high grade GIS GVHD in 10 days. The initial day of GVHD, days to extending to GIS GVHD and progressing to high grade did not influence the mortality. Melphalan and ATG usage in conditioning regimen and the drugs used in GVHD prophylaxis were not associated with survival. Low albumin level at any time of the severe GIS GVHD was associated with high mortality possibly due to more inflammatory GIS state of lost patients. Because ATG usage in the treatment of GIS GVHD significantly increased the mortality in our study, it should not be used in a routine manner. Better but not significant outcome with non-immunosuppressants seems a better treatment approach.Today best approach to GIS GVHD is to prevent and recognize it early. Treatment without immunosuppressive therapy like Photopheresis and mesenchymal stem cells seems a better approach which deserves further research with more patients.
Session topic: E-poster
Keyword(s): Graft-versus-host disease (GVHD), Hematopoietic cell transplantation, Pediatric
Type: Publication Only
Background
The high grade acute graft versus host disease (aGVHD) of typically involved organs (skin, gastrointestinal system (GIS) and liver) have specific features of therapeutic challenges, among which high grade GIS aGVHD has a distinctive and remarkable place in this context.
Aims
Our study intend to bring out the risk factors and clinical course of high grade gastrointestinal system (GIS) GVHD in children.
Methods
This is a retrospective analysis of 28 pediatric patients presented with a clinical diagnosis of stage 3 and 4 acute GVHD of the GIS who were selected from allogeneic hematopoetic stem cell transplantation (HSCT) performed. The demographics, the regimen used for conditioning and GVHD prophylaxis, clinical characteristics of GVHD including follow-up, laboratory parameters during GVHD, treatment modalities used for GVHD, response assessment in every week, complications of GVHD, and survival data were recorded.
Results
Patient and transplant characteristics were summarized in Table 1.
Conclusion
Overall survival at 3 months after the onset of stage 3 or 4 gut GVHD was 54%. Better outcome than the adult data might be related with more usage of bone marrow as a stem cell source or different characteristics of pediatric gastrointestinal system. In general, GVHD started 14 days post-HSCT, extended to GIS GVHD in a week and progressed to high grade GIS GVHD in 10 days. The initial day of GVHD, days to extending to GIS GVHD and progressing to high grade did not influence the mortality. Melphalan and ATG usage in conditioning regimen and the drugs used in GVHD prophylaxis were not associated with survival. Low albumin level at any time of the severe GIS GVHD was associated with high mortality possibly due to more inflammatory GIS state of lost patients. Because ATG usage in the treatment of GIS GVHD significantly increased the mortality in our study, it should not be used in a routine manner. Better but not significant outcome with non-immunosuppressants seems a better treatment approach.Today best approach to GIS GVHD is to prevent and recognize it early. Treatment without immunosuppressive therapy like Photopheresis and mesenchymal stem cells seems a better approach which deserves further research with more patients.

Session topic: E-poster
Keyword(s): Graft-versus-host disease (GVHD), Hematopoietic cell transplantation, Pediatric
Abstract: PB2156
Type: Publication Only
Background
The high grade acute graft versus host disease (aGVHD) of typically involved organs (skin, gastrointestinal system (GIS) and liver) have specific features of therapeutic challenges, among which high grade GIS aGVHD has a distinctive and remarkable place in this context.
Aims
Our study intend to bring out the risk factors and clinical course of high grade gastrointestinal system (GIS) GVHD in children.
Methods
This is a retrospective analysis of 28 pediatric patients presented with a clinical diagnosis of stage 3 and 4 acute GVHD of the GIS who were selected from allogeneic hematopoetic stem cell transplantation (HSCT) performed. The demographics, the regimen used for conditioning and GVHD prophylaxis, clinical characteristics of GVHD including follow-up, laboratory parameters during GVHD, treatment modalities used for GVHD, response assessment in every week, complications of GVHD, and survival data were recorded.
Results
Patient and transplant characteristics were summarized in Table 1.
Conclusion
Overall survival at 3 months after the onset of stage 3 or 4 gut GVHD was 54%. Better outcome than the adult data might be related with more usage of bone marrow as a stem cell source or different characteristics of pediatric gastrointestinal system. In general, GVHD started 14 days post-HSCT, extended to GIS GVHD in a week and progressed to high grade GIS GVHD in 10 days. The initial day of GVHD, days to extending to GIS GVHD and progressing to high grade did not influence the mortality. Melphalan and ATG usage in conditioning regimen and the drugs used in GVHD prophylaxis were not associated with survival. Low albumin level at any time of the severe GIS GVHD was associated with high mortality possibly due to more inflammatory GIS state of lost patients. Because ATG usage in the treatment of GIS GVHD significantly increased the mortality in our study, it should not be used in a routine manner. Better but not significant outcome with non-immunosuppressants seems a better treatment approach.Today best approach to GIS GVHD is to prevent and recognize it early. Treatment without immunosuppressive therapy like Photopheresis and mesenchymal stem cells seems a better approach which deserves further research with more patients.

Session topic: E-poster
Keyword(s): Graft-versus-host disease (GVHD), Hematopoietic cell transplantation, Pediatric
Type: Publication Only
Background
The high grade acute graft versus host disease (aGVHD) of typically involved organs (skin, gastrointestinal system (GIS) and liver) have specific features of therapeutic challenges, among which high grade GIS aGVHD has a distinctive and remarkable place in this context.
Aims
Our study intend to bring out the risk factors and clinical course of high grade gastrointestinal system (GIS) GVHD in children.
Methods
This is a retrospective analysis of 28 pediatric patients presented with a clinical diagnosis of stage 3 and 4 acute GVHD of the GIS who were selected from allogeneic hematopoetic stem cell transplantation (HSCT) performed. The demographics, the regimen used for conditioning and GVHD prophylaxis, clinical characteristics of GVHD including follow-up, laboratory parameters during GVHD, treatment modalities used for GVHD, response assessment in every week, complications of GVHD, and survival data were recorded.
Results
Patient and transplant characteristics were summarized in Table 1.
Conclusion
Overall survival at 3 months after the onset of stage 3 or 4 gut GVHD was 54%. Better outcome than the adult data might be related with more usage of bone marrow as a stem cell source or different characteristics of pediatric gastrointestinal system. In general, GVHD started 14 days post-HSCT, extended to GIS GVHD in a week and progressed to high grade GIS GVHD in 10 days. The initial day of GVHD, days to extending to GIS GVHD and progressing to high grade did not influence the mortality. Melphalan and ATG usage in conditioning regimen and the drugs used in GVHD prophylaxis were not associated with survival. Low albumin level at any time of the severe GIS GVHD was associated with high mortality possibly due to more inflammatory GIS state of lost patients. Because ATG usage in the treatment of GIS GVHD significantly increased the mortality in our study, it should not be used in a routine manner. Better but not significant outcome with non-immunosuppressants seems a better treatment approach.Today best approach to GIS GVHD is to prevent and recognize it early. Treatment without immunosuppressive therapy like Photopheresis and mesenchymal stem cells seems a better approach which deserves further research with more patients.

Session topic: E-poster
Keyword(s): Graft-versus-host disease (GVHD), Hematopoietic cell transplantation, Pediatric
{{ help_message }}
{{filter}}


