HIGH-SENSITIVE MONITORING OF CHIMERISM IN BLOOD AFTER HAEMATOPOIETIC STEM CELL TRANSPLANTATION IN CHILDHOOD LEUKAEMIA
(Abstract release date: 05/19/16)
EHA Library. Haugaard A. 06/09/16; 135051; PB2151

Ms. Anna Karen Haugaard
Contributions
Contributions
Abstract
Abstract: PB2151
Type: Publication Only
Background
We hereby report from a study aiming to evaluate the ability of microchimerism to predict relapse in a retrospective Danish cohort. Final results are pending.Relapse is the primary treatment failure after haematopoietic stem cell transplantation (HCT) for acute leukaemia in childhood, with a cumulative risk of 20-35%. Early detection of increasing minimal residual disease (MRD) or recipient chimerism is increasingly important to monitor imminent post HCT relapse. Chimerism analysis by polymerase chain reaction (PCR) of short tandem repeats is the gold standard and has a sensitivity of 1-5%. However, chimerism analysis based on real time, quantitative PCR (RQ-PCR) analysis (microchimerism) is more sensitive, with a reported detection level of 0.1-0.01%. We aimed to evaluate the ability of microchimerism to predict relapse in a retrospective Danish paediatric cohort.
Aims
It is the purpose of this study to evaluate the ability of microchimerism to predict early relapse following allogeneic SCT in children with leukaemia, with clinical and molecular relapse as primary outcome parameters. Microchimerism will be compared to results from detection of MRD in bone marrow and to standard STR-PCR based chimerism analysis.
Methods
Children transplanted for acute lymphoid (ALL) or acute myeloid leukaemia (AML), between 2008 and 2014 at the Department for Children and Adolescents, University Hospital of Copenhagen, were included. Microchimerism was analysed on peripheral blood DNA using a commercially available kit (GenDx), based on allele-specific RQ- PCR of insertions/deletion polymorphisms. Results were compared with bone marrow MRD analysis and with standard, peripheral blood (PB) chimerism in DNA from unseparated whole blood, and PB cells positive for CD66b, CD4 and CD8. Transplant related data was collected from the European society for Blood and Marrow Transplantation (EBMT) database. We defined complete chimerism (CC) as no detectable recipient-cell chimerism at any time point, stable mixed chimerism (SMC) as a single increase below upper sensitivity limit, and increasing mixed chimerism (IMC) as two or more increases or a single increase above upper sensitivity limit.
Results
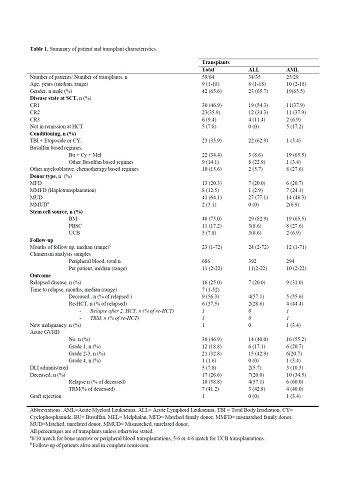
We included 59 children (ALL n=34, AML n=25), seven of these were re-transplanted due to relapse or rejection. Two transplants were excluded from further analysis due to lack of available DNA. Median age at HCT was 9 (1-8) years, and median follow up time was 23 (1-72) months. In total, 686 samples of frozen DNA were available for analysis, with a median (range) of 11 (2-22) samples per transplant. HCT was performed in 1st complete remission (CR) in 30 (47%), 2nd CR in 23 (36%), 3rd CR in 6 (%) and five (8%) performed without remission. Transplant characteristics and outcome data are displayed in Table 1.Seven children (21%) with ALL and 9 (36%) with AML relapsed, a median of 7 (1-52) months from HCT. One child re-transplanted in second remission subsequently relapsed again. In total, seventeen (29%) children died a median of 266 (60-1391) days from latest HCT, 10 (59%) of relapsed disease and 7 (41%) from transplant-related causes.For standard chimerism, 12/16 with IMC relapsed, versus 2/16 and 3/25 with SMC and CC, respectively. Cumulative incidence between the groups SMC, CC and IMC was statistically different for relapse (P<0.0001), and remained so after grouping CC and SMC.
Conclusion
Final results of microchimerism analysis are currently pending and will be presented at the EHA conference.
Session topic: E-poster
Keyword(s): Chimerism, HSCT, Leukemia, Pediatric
Type: Publication Only
Background
We hereby report from a study aiming to evaluate the ability of microchimerism to predict relapse in a retrospective Danish cohort. Final results are pending.Relapse is the primary treatment failure after haematopoietic stem cell transplantation (HCT) for acute leukaemia in childhood, with a cumulative risk of 20-35%. Early detection of increasing minimal residual disease (MRD) or recipient chimerism is increasingly important to monitor imminent post HCT relapse. Chimerism analysis by polymerase chain reaction (PCR) of short tandem repeats is the gold standard and has a sensitivity of 1-5%. However, chimerism analysis based on real time, quantitative PCR (RQ-PCR) analysis (microchimerism) is more sensitive, with a reported detection level of 0.1-0.01%. We aimed to evaluate the ability of microchimerism to predict relapse in a retrospective Danish paediatric cohort.
Aims
It is the purpose of this study to evaluate the ability of microchimerism to predict early relapse following allogeneic SCT in children with leukaemia, with clinical and molecular relapse as primary outcome parameters. Microchimerism will be compared to results from detection of MRD in bone marrow and to standard STR-PCR based chimerism analysis.
Methods
Children transplanted for acute lymphoid (ALL) or acute myeloid leukaemia (AML), between 2008 and 2014 at the Department for Children and Adolescents, University Hospital of Copenhagen, were included. Microchimerism was analysed on peripheral blood DNA using a commercially available kit (GenDx), based on allele-specific RQ- PCR of insertions/deletion polymorphisms. Results were compared with bone marrow MRD analysis and with standard, peripheral blood (PB) chimerism in DNA from unseparated whole blood, and PB cells positive for CD66b, CD4 and CD8. Transplant related data was collected from the European society for Blood and Marrow Transplantation (EBMT) database. We defined complete chimerism (CC) as no detectable recipient-cell chimerism at any time point, stable mixed chimerism (SMC) as a single increase below upper sensitivity limit, and increasing mixed chimerism (IMC) as two or more increases or a single increase above upper sensitivity limit.
Results
We included 59 children (ALL n=34, AML n=25), seven of these were re-transplanted due to relapse or rejection. Two transplants were excluded from further analysis due to lack of available DNA. Median age at HCT was 9 (1-8) years, and median follow up time was 23 (1-72) months. In total, 686 samples of frozen DNA were available for analysis, with a median (range) of 11 (2-22) samples per transplant. HCT was performed in 1st complete remission (CR) in 30 (47%), 2nd CR in 23 (36%), 3rd CR in 6 (%) and five (8%) performed without remission. Transplant characteristics and outcome data are displayed in Table 1.Seven children (21%) with ALL and 9 (36%) with AML relapsed, a median of 7 (1-52) months from HCT. One child re-transplanted in second remission subsequently relapsed again. In total, seventeen (29%) children died a median of 266 (60-1391) days from latest HCT, 10 (59%) of relapsed disease and 7 (41%) from transplant-related causes.For standard chimerism, 12/16 with IMC relapsed, versus 2/16 and 3/25 with SMC and CC, respectively. Cumulative incidence between the groups SMC, CC and IMC was statistically different for relapse (P<0.0001), and remained so after grouping CC and SMC.
Conclusion
Final results of microchimerism analysis are currently pending and will be presented at the EHA conference.

Session topic: E-poster
Keyword(s): Chimerism, HSCT, Leukemia, Pediatric
Abstract: PB2151
Type: Publication Only
Background
We hereby report from a study aiming to evaluate the ability of microchimerism to predict relapse in a retrospective Danish cohort. Final results are pending.Relapse is the primary treatment failure after haematopoietic stem cell transplantation (HCT) for acute leukaemia in childhood, with a cumulative risk of 20-35%. Early detection of increasing minimal residual disease (MRD) or recipient chimerism is increasingly important to monitor imminent post HCT relapse. Chimerism analysis by polymerase chain reaction (PCR) of short tandem repeats is the gold standard and has a sensitivity of 1-5%. However, chimerism analysis based on real time, quantitative PCR (RQ-PCR) analysis (microchimerism) is more sensitive, with a reported detection level of 0.1-0.01%. We aimed to evaluate the ability of microchimerism to predict relapse in a retrospective Danish paediatric cohort.
Aims
It is the purpose of this study to evaluate the ability of microchimerism to predict early relapse following allogeneic SCT in children with leukaemia, with clinical and molecular relapse as primary outcome parameters. Microchimerism will be compared to results from detection of MRD in bone marrow and to standard STR-PCR based chimerism analysis.
Methods
Children transplanted for acute lymphoid (ALL) or acute myeloid leukaemia (AML), between 2008 and 2014 at the Department for Children and Adolescents, University Hospital of Copenhagen, were included. Microchimerism was analysed on peripheral blood DNA using a commercially available kit (GenDx), based on allele-specific RQ- PCR of insertions/deletion polymorphisms. Results were compared with bone marrow MRD analysis and with standard, peripheral blood (PB) chimerism in DNA from unseparated whole blood, and PB cells positive for CD66b, CD4 and CD8. Transplant related data was collected from the European society for Blood and Marrow Transplantation (EBMT) database. We defined complete chimerism (CC) as no detectable recipient-cell chimerism at any time point, stable mixed chimerism (SMC) as a single increase below upper sensitivity limit, and increasing mixed chimerism (IMC) as two or more increases or a single increase above upper sensitivity limit.
Results
We included 59 children (ALL n=34, AML n=25), seven of these were re-transplanted due to relapse or rejection. Two transplants were excluded from further analysis due to lack of available DNA. Median age at HCT was 9 (1-8) years, and median follow up time was 23 (1-72) months. In total, 686 samples of frozen DNA were available for analysis, with a median (range) of 11 (2-22) samples per transplant. HCT was performed in 1st complete remission (CR) in 30 (47%), 2nd CR in 23 (36%), 3rd CR in 6 (%) and five (8%) performed without remission. Transplant characteristics and outcome data are displayed in Table 1.Seven children (21%) with ALL and 9 (36%) with AML relapsed, a median of 7 (1-52) months from HCT. One child re-transplanted in second remission subsequently relapsed again. In total, seventeen (29%) children died a median of 266 (60-1391) days from latest HCT, 10 (59%) of relapsed disease and 7 (41%) from transplant-related causes.For standard chimerism, 12/16 with IMC relapsed, versus 2/16 and 3/25 with SMC and CC, respectively. Cumulative incidence between the groups SMC, CC and IMC was statistically different for relapse (P<0.0001), and remained so after grouping CC and SMC.
Conclusion
Final results of microchimerism analysis are currently pending and will be presented at the EHA conference.

Session topic: E-poster
Keyword(s): Chimerism, HSCT, Leukemia, Pediatric
Type: Publication Only
Background
We hereby report from a study aiming to evaluate the ability of microchimerism to predict relapse in a retrospective Danish cohort. Final results are pending.Relapse is the primary treatment failure after haematopoietic stem cell transplantation (HCT) for acute leukaemia in childhood, with a cumulative risk of 20-35%. Early detection of increasing minimal residual disease (MRD) or recipient chimerism is increasingly important to monitor imminent post HCT relapse. Chimerism analysis by polymerase chain reaction (PCR) of short tandem repeats is the gold standard and has a sensitivity of 1-5%. However, chimerism analysis based on real time, quantitative PCR (RQ-PCR) analysis (microchimerism) is more sensitive, with a reported detection level of 0.1-0.01%. We aimed to evaluate the ability of microchimerism to predict relapse in a retrospective Danish paediatric cohort.
Aims
It is the purpose of this study to evaluate the ability of microchimerism to predict early relapse following allogeneic SCT in children with leukaemia, with clinical and molecular relapse as primary outcome parameters. Microchimerism will be compared to results from detection of MRD in bone marrow and to standard STR-PCR based chimerism analysis.
Methods
Children transplanted for acute lymphoid (ALL) or acute myeloid leukaemia (AML), between 2008 and 2014 at the Department for Children and Adolescents, University Hospital of Copenhagen, were included. Microchimerism was analysed on peripheral blood DNA using a commercially available kit (GenDx), based on allele-specific RQ- PCR of insertions/deletion polymorphisms. Results were compared with bone marrow MRD analysis and with standard, peripheral blood (PB) chimerism in DNA from unseparated whole blood, and PB cells positive for CD66b, CD4 and CD8. Transplant related data was collected from the European society for Blood and Marrow Transplantation (EBMT) database. We defined complete chimerism (CC) as no detectable recipient-cell chimerism at any time point, stable mixed chimerism (SMC) as a single increase below upper sensitivity limit, and increasing mixed chimerism (IMC) as two or more increases or a single increase above upper sensitivity limit.
Results
We included 59 children (ALL n=34, AML n=25), seven of these were re-transplanted due to relapse or rejection. Two transplants were excluded from further analysis due to lack of available DNA. Median age at HCT was 9 (1-8) years, and median follow up time was 23 (1-72) months. In total, 686 samples of frozen DNA were available for analysis, with a median (range) of 11 (2-22) samples per transplant. HCT was performed in 1st complete remission (CR) in 30 (47%), 2nd CR in 23 (36%), 3rd CR in 6 (%) and five (8%) performed without remission. Transplant characteristics and outcome data are displayed in Table 1.Seven children (21%) with ALL and 9 (36%) with AML relapsed, a median of 7 (1-52) months from HCT. One child re-transplanted in second remission subsequently relapsed again. In total, seventeen (29%) children died a median of 266 (60-1391) days from latest HCT, 10 (59%) of relapsed disease and 7 (41%) from transplant-related causes.For standard chimerism, 12/16 with IMC relapsed, versus 2/16 and 3/25 with SMC and CC, respectively. Cumulative incidence between the groups SMC, CC and IMC was statistically different for relapse (P<0.0001), and remained so after grouping CC and SMC.
Conclusion
Final results of microchimerism analysis are currently pending and will be presented at the EHA conference.

Session topic: E-poster
Keyword(s): Chimerism, HSCT, Leukemia, Pediatric
{{ help_message }}
{{filter}}


