CHELATION THERAPY IN NON-TRANSFUSION-DEPENDENT IRON LOADING ANEMIA. EXPERIENCE IN A TERTIARY HOSPITAL.
(Abstract release date: 05/19/16)
EHA Library. Del Orbe Barreto R. 06/09/16; 135047; PB2147

Dr. Rafael Del Orbe Barreto
Contributions
Contributions
Abstract
Abstract: PB2147
Type: Publication Only
Background
Iron loading anemia (ILA) encompasses a group of inherited and acquired anemias characterized by ineffective erythropoiesis, low hepcidin levels, excessive intestinal iron absorption and secondary iron overload. This iron accumulation occurs more slowly compared to transfusion-dependent patients, and complications do not arise until later in life. It remains crucial monitoring and appropriately treat their iron burden, for this reason it is essential to manage it appropriately with iron chelation therapy, especially since phlebotomy is not an option in these patients.
Aims
To describe the clinical management of chelation therapy in patients with non-transfusion-dependent iron loading anemia.
Methods
This retrospective study was conducted at Cruces University Hospital in Barakaldo – Spain, from February 2009 to February 2016. Clinical data and results of Hemoglobin, serum ferritin and transferrin saturation, Liver Iron Concentration (LIC) and Cardiac T2* measured by MRI was obtained from patient´s medical records. All patients were treated with Deferasirox 500 mg/day as compassionate use since none was transfused. Safety profile was studied in terms of gastrointestinal side effects, rash and change in serum creatinine and AST-ALT values.
Results
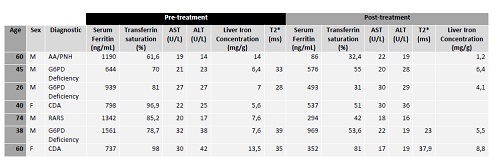
Seven patients were included (5 male, 2 female). Medium age was 57 years (min 26, max 74). 1 patient was diagnosed with non-severe Aplastic anemia/Paroxysmal nocturnal hemoglobinuria (AA/PNH), 3 with Glucose-6-phosphate dehydrogenase (G6PD) deficiency, 2 Congenital Dyseritropoietic Anemia (CDA) and 1 Refractory anemia with ring sideroblasts (RARS). None was splenectomized. Medium serum ferritin before treatment was 1109,5 ng/mL (min 644, max 1561) and after at least 12 months post-treatment was 538 ng/mL (min 86, max 969). In 5 patients LIC was evaluated after treatment, in 4 of them a reduction was observed. At baseline 4 patients were evaluated with T2* and anyone showed cardiac iron overload. No adverse events were observed in any patient.
Conclusion
There are currently no standard clinical practice guidelines for the treatment of iron overload in Iron Loading Anemia. In our experience, using Deferasirox in this kind of patients is a useful and safely strategy to manage Iron overload. Prospective studies are needed to evaluate efficacy and safety of chelation therapy in this setting.
Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload
Type: Publication Only
Background
Iron loading anemia (ILA) encompasses a group of inherited and acquired anemias characterized by ineffective erythropoiesis, low hepcidin levels, excessive intestinal iron absorption and secondary iron overload. This iron accumulation occurs more slowly compared to transfusion-dependent patients, and complications do not arise until later in life. It remains crucial monitoring and appropriately treat their iron burden, for this reason it is essential to manage it appropriately with iron chelation therapy, especially since phlebotomy is not an option in these patients.
Aims
To describe the clinical management of chelation therapy in patients with non-transfusion-dependent iron loading anemia.
Methods
This retrospective study was conducted at Cruces University Hospital in Barakaldo – Spain, from February 2009 to February 2016. Clinical data and results of Hemoglobin, serum ferritin and transferrin saturation, Liver Iron Concentration (LIC) and Cardiac T2* measured by MRI was obtained from patient´s medical records. All patients were treated with Deferasirox 500 mg/day as compassionate use since none was transfused. Safety profile was studied in terms of gastrointestinal side effects, rash and change in serum creatinine and AST-ALT values.
Results
Seven patients were included (5 male, 2 female). Medium age was 57 years (min 26, max 74). 1 patient was diagnosed with non-severe Aplastic anemia/Paroxysmal nocturnal hemoglobinuria (AA/PNH), 3 with Glucose-6-phosphate dehydrogenase (G6PD) deficiency, 2 Congenital Dyseritropoietic Anemia (CDA) and 1 Refractory anemia with ring sideroblasts (RARS). None was splenectomized. Medium serum ferritin before treatment was 1109,5 ng/mL (min 644, max 1561) and after at least 12 months post-treatment was 538 ng/mL (min 86, max 969). In 5 patients LIC was evaluated after treatment, in 4 of them a reduction was observed. At baseline 4 patients were evaluated with T2* and anyone showed cardiac iron overload. No adverse events were observed in any patient.
Conclusion
There are currently no standard clinical practice guidelines for the treatment of iron overload in Iron Loading Anemia. In our experience, using Deferasirox in this kind of patients is a useful and safely strategy to manage Iron overload. Prospective studies are needed to evaluate efficacy and safety of chelation therapy in this setting.

Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload
Abstract: PB2147
Type: Publication Only
Background
Iron loading anemia (ILA) encompasses a group of inherited and acquired anemias characterized by ineffective erythropoiesis, low hepcidin levels, excessive intestinal iron absorption and secondary iron overload. This iron accumulation occurs more slowly compared to transfusion-dependent patients, and complications do not arise until later in life. It remains crucial monitoring and appropriately treat their iron burden, for this reason it is essential to manage it appropriately with iron chelation therapy, especially since phlebotomy is not an option in these patients.
Aims
To describe the clinical management of chelation therapy in patients with non-transfusion-dependent iron loading anemia.
Methods
This retrospective study was conducted at Cruces University Hospital in Barakaldo – Spain, from February 2009 to February 2016. Clinical data and results of Hemoglobin, serum ferritin and transferrin saturation, Liver Iron Concentration (LIC) and Cardiac T2* measured by MRI was obtained from patient´s medical records. All patients were treated with Deferasirox 500 mg/day as compassionate use since none was transfused. Safety profile was studied in terms of gastrointestinal side effects, rash and change in serum creatinine and AST-ALT values.
Results
Seven patients were included (5 male, 2 female). Medium age was 57 years (min 26, max 74). 1 patient was diagnosed with non-severe Aplastic anemia/Paroxysmal nocturnal hemoglobinuria (AA/PNH), 3 with Glucose-6-phosphate dehydrogenase (G6PD) deficiency, 2 Congenital Dyseritropoietic Anemia (CDA) and 1 Refractory anemia with ring sideroblasts (RARS). None was splenectomized. Medium serum ferritin before treatment was 1109,5 ng/mL (min 644, max 1561) and after at least 12 months post-treatment was 538 ng/mL (min 86, max 969). In 5 patients LIC was evaluated after treatment, in 4 of them a reduction was observed. At baseline 4 patients were evaluated with T2* and anyone showed cardiac iron overload. No adverse events were observed in any patient.
Conclusion
There are currently no standard clinical practice guidelines for the treatment of iron overload in Iron Loading Anemia. In our experience, using Deferasirox in this kind of patients is a useful and safely strategy to manage Iron overload. Prospective studies are needed to evaluate efficacy and safety of chelation therapy in this setting.

Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload
Type: Publication Only
Background
Iron loading anemia (ILA) encompasses a group of inherited and acquired anemias characterized by ineffective erythropoiesis, low hepcidin levels, excessive intestinal iron absorption and secondary iron overload. This iron accumulation occurs more slowly compared to transfusion-dependent patients, and complications do not arise until later in life. It remains crucial monitoring and appropriately treat their iron burden, for this reason it is essential to manage it appropriately with iron chelation therapy, especially since phlebotomy is not an option in these patients.
Aims
To describe the clinical management of chelation therapy in patients with non-transfusion-dependent iron loading anemia.
Methods
This retrospective study was conducted at Cruces University Hospital in Barakaldo – Spain, from February 2009 to February 2016. Clinical data and results of Hemoglobin, serum ferritin and transferrin saturation, Liver Iron Concentration (LIC) and Cardiac T2* measured by MRI was obtained from patient´s medical records. All patients were treated with Deferasirox 500 mg/day as compassionate use since none was transfused. Safety profile was studied in terms of gastrointestinal side effects, rash and change in serum creatinine and AST-ALT values.
Results
Seven patients were included (5 male, 2 female). Medium age was 57 years (min 26, max 74). 1 patient was diagnosed with non-severe Aplastic anemia/Paroxysmal nocturnal hemoglobinuria (AA/PNH), 3 with Glucose-6-phosphate dehydrogenase (G6PD) deficiency, 2 Congenital Dyseritropoietic Anemia (CDA) and 1 Refractory anemia with ring sideroblasts (RARS). None was splenectomized. Medium serum ferritin before treatment was 1109,5 ng/mL (min 644, max 1561) and after at least 12 months post-treatment was 538 ng/mL (min 86, max 969). In 5 patients LIC was evaluated after treatment, in 4 of them a reduction was observed. At baseline 4 patients were evaluated with T2* and anyone showed cardiac iron overload. No adverse events were observed in any patient.
Conclusion
There are currently no standard clinical practice guidelines for the treatment of iron overload in Iron Loading Anemia. In our experience, using Deferasirox in this kind of patients is a useful and safely strategy to manage Iron overload. Prospective studies are needed to evaluate efficacy and safety of chelation therapy in this setting.

Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload
{{ help_message }}
{{filter}}


