HYPERFERRITINEMIA: CLINICAL COURSE AND THERAPEUTIC STRATEGY
(Abstract release date: 05/19/16)
EHA Library. Papadopoulos V. 06/09/16; 135035; PB2135

Vassilios Papadopoulos
Contributions
Contributions
Abstract
Abstract: PB2135
Type: Publication Only
Background
Hyperferritinemia is a common laboratory finding, which requires assessment of iron status and investigation for underlying disorders, such as hereditary hemochromatosis, inflammatory and malignant conditions. True iron overload is found in approximately 10% of patients presenting with hyperferritinemia (Adams & Barton. Journal of Hepatology 2011;453-8). Although research of the last decades has given insight into new molecules and mutations and their role in iron homeostasis, the clinical consequences of all these mutations are not straighforward, and there are still cases of hyperferritinemia with no underlying cause identified.
Aims
Objective of the study is to assess the clinical course of patients with hyperferritinemia and the therapeutic manoeuvres performed.
Methods
Data was collected retrospectively for 17 patients with hyperferritinemia, followed up for a mean of 5.75 years. They were negative for classic HFE mutations (C282Y homozygosity, C282Y/H63D compound heterozygosity) and had no underlying malignancy, inflammation or alcohol abuse. Hepatic iron was assessed with MRI T2* or liver biopsy. The decision to propose therapeutic phlebotomy was based on presence of iron overload, eligibility for blood donation and patient preferences.
Results
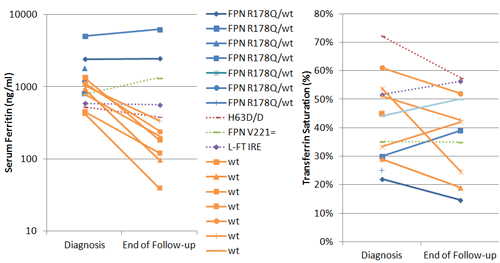
Seven patients were heterozygous for ferroportin (FPN) R178Q mutation. At diagnosis their mean serum ferritin level (SF) was 2,017ng/ml (range 800-5077) and trasferrin saturation (TS) was normal. Three patients with moderate iron overload were offered regular phlebotomies once per month; iron overload was diminished, while SF did not change significantly. In the maintainance phase, phlebotomies were performed once in 3 months, and interrupted during pregnancy and puerperium. Patients without iron overload were encouraged to become blood donors.Seven patients, albeit negative for tested HFE and ferroportin mutations, had mild to moderate hepatic iron accumulation, with mean SF at 880ng/ml (range 435-1351); three of them had elevated TS, that is over 45%, and two had fatty liver on imaging studies. Venesections were performed to all of them, once in 1-2months initially and then every 3-6 months. Iron accumulation and SF were reduced even down to normal ranges; venesections were well tolerated at the aforementioned frequency.A patient homozygous for HFE-H63D mutation, with high SF and high TS, had no iron overload; SF was reduced without any intervention during the follow-up. A teenage girl with ferroportin polymorphism had isolated hyperferritinemia without iron overload nor high TS, which remained so after 8 years.A patient with hyperferritinemia, high TS and catarract, was tested positive for the light ferritin 5’-UTR iron response element (L-FT IRE) mutation, which has been identified in the so-called ‘hereditary hyperferritinemia-catarract syndrome’. No iron overload was detected at diagnosis and after 5 years of follow-up.These changes of SF and TS from baseline values are depicted in the figure (blue lines: FPN heterozygotes, orange lines[wt]:no mutation identified).
Conclusion
In all of the described cases, a benign course of the patients with hyperferritinemia is evident. Even in the cases with moderate hepatic iron overload, a less stringent phlebotomies program (once per 1-2 months, compared to higher frequency of weekly venesections, recommended for classic and juvenile hereditary hemochromatosis types by European Association for the Study of the Liver, Journal of Hepatology 2010;53:3–22) seems to provide satisfactory response, removing excess iron from tissues and being very well tolerated. This proposal could be incorporated into prospective trials, in order for clear evidence-based guidelines to be developed in the future.
Session topic: E-poster
Keyword(s): Ferritin, Hemochromatosis, Iron overload
Type: Publication Only
Background
Hyperferritinemia is a common laboratory finding, which requires assessment of iron status and investigation for underlying disorders, such as hereditary hemochromatosis, inflammatory and malignant conditions. True iron overload is found in approximately 10% of patients presenting with hyperferritinemia (Adams & Barton. Journal of Hepatology 2011;453-8). Although research of the last decades has given insight into new molecules and mutations and their role in iron homeostasis, the clinical consequences of all these mutations are not straighforward, and there are still cases of hyperferritinemia with no underlying cause identified.
Aims
Objective of the study is to assess the clinical course of patients with hyperferritinemia and the therapeutic manoeuvres performed.
Methods
Data was collected retrospectively for 17 patients with hyperferritinemia, followed up for a mean of 5.75 years. They were negative for classic HFE mutations (C282Y homozygosity, C282Y/H63D compound heterozygosity) and had no underlying malignancy, inflammation or alcohol abuse. Hepatic iron was assessed with MRI T2* or liver biopsy. The decision to propose therapeutic phlebotomy was based on presence of iron overload, eligibility for blood donation and patient preferences.
Results
Seven patients were heterozygous for ferroportin (FPN) R178Q mutation. At diagnosis their mean serum ferritin level (SF) was 2,017ng/ml (range 800-5077) and trasferrin saturation (TS) was normal. Three patients with moderate iron overload were offered regular phlebotomies once per month; iron overload was diminished, while SF did not change significantly. In the maintainance phase, phlebotomies were performed once in 3 months, and interrupted during pregnancy and puerperium. Patients without iron overload were encouraged to become blood donors.Seven patients, albeit negative for tested HFE and ferroportin mutations, had mild to moderate hepatic iron accumulation, with mean SF at 880ng/ml (range 435-1351); three of them had elevated TS, that is over 45%, and two had fatty liver on imaging studies. Venesections were performed to all of them, once in 1-2months initially and then every 3-6 months. Iron accumulation and SF were reduced even down to normal ranges; venesections were well tolerated at the aforementioned frequency.A patient homozygous for HFE-H63D mutation, with high SF and high TS, had no iron overload; SF was reduced without any intervention during the follow-up. A teenage girl with ferroportin polymorphism had isolated hyperferritinemia without iron overload nor high TS, which remained so after 8 years.A patient with hyperferritinemia, high TS and catarract, was tested positive for the light ferritin 5’-UTR iron response element (L-FT IRE) mutation, which has been identified in the so-called ‘hereditary hyperferritinemia-catarract syndrome’. No iron overload was detected at diagnosis and after 5 years of follow-up.These changes of SF and TS from baseline values are depicted in the figure (blue lines: FPN heterozygotes, orange lines[wt]:no mutation identified).
Conclusion
In all of the described cases, a benign course of the patients with hyperferritinemia is evident. Even in the cases with moderate hepatic iron overload, a less stringent phlebotomies program (once per 1-2 months, compared to higher frequency of weekly venesections, recommended for classic and juvenile hereditary hemochromatosis types by European Association for the Study of the Liver, Journal of Hepatology 2010;53:3–22) seems to provide satisfactory response, removing excess iron from tissues and being very well tolerated. This proposal could be incorporated into prospective trials, in order for clear evidence-based guidelines to be developed in the future.

Session topic: E-poster
Keyword(s): Ferritin, Hemochromatosis, Iron overload
Abstract: PB2135
Type: Publication Only
Background
Hyperferritinemia is a common laboratory finding, which requires assessment of iron status and investigation for underlying disorders, such as hereditary hemochromatosis, inflammatory and malignant conditions. True iron overload is found in approximately 10% of patients presenting with hyperferritinemia (Adams & Barton. Journal of Hepatology 2011;453-8). Although research of the last decades has given insight into new molecules and mutations and their role in iron homeostasis, the clinical consequences of all these mutations are not straighforward, and there are still cases of hyperferritinemia with no underlying cause identified.
Aims
Objective of the study is to assess the clinical course of patients with hyperferritinemia and the therapeutic manoeuvres performed.
Methods
Data was collected retrospectively for 17 patients with hyperferritinemia, followed up for a mean of 5.75 years. They were negative for classic HFE mutations (C282Y homozygosity, C282Y/H63D compound heterozygosity) and had no underlying malignancy, inflammation or alcohol abuse. Hepatic iron was assessed with MRI T2* or liver biopsy. The decision to propose therapeutic phlebotomy was based on presence of iron overload, eligibility for blood donation and patient preferences.
Results
Seven patients were heterozygous for ferroportin (FPN) R178Q mutation. At diagnosis their mean serum ferritin level (SF) was 2,017ng/ml (range 800-5077) and trasferrin saturation (TS) was normal. Three patients with moderate iron overload were offered regular phlebotomies once per month; iron overload was diminished, while SF did not change significantly. In the maintainance phase, phlebotomies were performed once in 3 months, and interrupted during pregnancy and puerperium. Patients without iron overload were encouraged to become blood donors.Seven patients, albeit negative for tested HFE and ferroportin mutations, had mild to moderate hepatic iron accumulation, with mean SF at 880ng/ml (range 435-1351); three of them had elevated TS, that is over 45%, and two had fatty liver on imaging studies. Venesections were performed to all of them, once in 1-2months initially and then every 3-6 months. Iron accumulation and SF were reduced even down to normal ranges; venesections were well tolerated at the aforementioned frequency.A patient homozygous for HFE-H63D mutation, with high SF and high TS, had no iron overload; SF was reduced without any intervention during the follow-up. A teenage girl with ferroportin polymorphism had isolated hyperferritinemia without iron overload nor high TS, which remained so after 8 years.A patient with hyperferritinemia, high TS and catarract, was tested positive for the light ferritin 5’-UTR iron response element (L-FT IRE) mutation, which has been identified in the so-called ‘hereditary hyperferritinemia-catarract syndrome’. No iron overload was detected at diagnosis and after 5 years of follow-up.These changes of SF and TS from baseline values are depicted in the figure (blue lines: FPN heterozygotes, orange lines[wt]:no mutation identified).
Conclusion
In all of the described cases, a benign course of the patients with hyperferritinemia is evident. Even in the cases with moderate hepatic iron overload, a less stringent phlebotomies program (once per 1-2 months, compared to higher frequency of weekly venesections, recommended for classic and juvenile hereditary hemochromatosis types by European Association for the Study of the Liver, Journal of Hepatology 2010;53:3–22) seems to provide satisfactory response, removing excess iron from tissues and being very well tolerated. This proposal could be incorporated into prospective trials, in order for clear evidence-based guidelines to be developed in the future.

Session topic: E-poster
Keyword(s): Ferritin, Hemochromatosis, Iron overload
Type: Publication Only
Background
Hyperferritinemia is a common laboratory finding, which requires assessment of iron status and investigation for underlying disorders, such as hereditary hemochromatosis, inflammatory and malignant conditions. True iron overload is found in approximately 10% of patients presenting with hyperferritinemia (Adams & Barton. Journal of Hepatology 2011;453-8). Although research of the last decades has given insight into new molecules and mutations and their role in iron homeostasis, the clinical consequences of all these mutations are not straighforward, and there are still cases of hyperferritinemia with no underlying cause identified.
Aims
Objective of the study is to assess the clinical course of patients with hyperferritinemia and the therapeutic manoeuvres performed.
Methods
Data was collected retrospectively for 17 patients with hyperferritinemia, followed up for a mean of 5.75 years. They were negative for classic HFE mutations (C282Y homozygosity, C282Y/H63D compound heterozygosity) and had no underlying malignancy, inflammation or alcohol abuse. Hepatic iron was assessed with MRI T2* or liver biopsy. The decision to propose therapeutic phlebotomy was based on presence of iron overload, eligibility for blood donation and patient preferences.
Results
Seven patients were heterozygous for ferroportin (FPN) R178Q mutation. At diagnosis their mean serum ferritin level (SF) was 2,017ng/ml (range 800-5077) and trasferrin saturation (TS) was normal. Three patients with moderate iron overload were offered regular phlebotomies once per month; iron overload was diminished, while SF did not change significantly. In the maintainance phase, phlebotomies were performed once in 3 months, and interrupted during pregnancy and puerperium. Patients without iron overload were encouraged to become blood donors.Seven patients, albeit negative for tested HFE and ferroportin mutations, had mild to moderate hepatic iron accumulation, with mean SF at 880ng/ml (range 435-1351); three of them had elevated TS, that is over 45%, and two had fatty liver on imaging studies. Venesections were performed to all of them, once in 1-2months initially and then every 3-6 months. Iron accumulation and SF were reduced even down to normal ranges; venesections were well tolerated at the aforementioned frequency.A patient homozygous for HFE-H63D mutation, with high SF and high TS, had no iron overload; SF was reduced without any intervention during the follow-up. A teenage girl with ferroportin polymorphism had isolated hyperferritinemia without iron overload nor high TS, which remained so after 8 years.A patient with hyperferritinemia, high TS and catarract, was tested positive for the light ferritin 5’-UTR iron response element (L-FT IRE) mutation, which has been identified in the so-called ‘hereditary hyperferritinemia-catarract syndrome’. No iron overload was detected at diagnosis and after 5 years of follow-up.These changes of SF and TS from baseline values are depicted in the figure (blue lines: FPN heterozygotes, orange lines[wt]:no mutation identified).
Conclusion
In all of the described cases, a benign course of the patients with hyperferritinemia is evident. Even in the cases with moderate hepatic iron overload, a less stringent phlebotomies program (once per 1-2 months, compared to higher frequency of weekly venesections, recommended for classic and juvenile hereditary hemochromatosis types by European Association for the Study of the Liver, Journal of Hepatology 2010;53:3–22) seems to provide satisfactory response, removing excess iron from tissues and being very well tolerated. This proposal could be incorporated into prospective trials, in order for clear evidence-based guidelines to be developed in the future.

Session topic: E-poster
Keyword(s): Ferritin, Hemochromatosis, Iron overload
{{ help_message }}
{{filter}}


