EVALUATION OF A MORE EFFECTIVE TREATMENT REGIMEN AND DOSE OF RECOMBINANT HUMAN ERYTHROPOIETIN FOR MEDICATION OF ANEMIA IN PREGNANCY
(Abstract release date: 05/19/16)
EHA Library. Demikhov V. 06/09/16; 135024; PB2124

Prof. Valerii Demikhov
Contributions
Contributions
Abstract
Abstract: PB2124
Type: Publication Only
Background
There is evidence that pathogenesis of anemia in pregnancy (AP) is multifactorial. It has not relation to ineffective erythropoiesis, caused by iron or folate deficiency only. Blunted erythropoiesis is one of main AP causes. According to our own data inadequately low production of erythropoietin (EPO) for the degree of the anemia found at more 50% of anemic pregnants. That is why recombinant human erythropoietin (rHuEPO) combined with iron is effective method in the therapy of anemic pregnant women, who had been ineffectively treated with iron alone. Because EPO does not cross the placenta, this protects the fetus from its use during pregnancy. Most recent studies show overall the effect of rHuEpo is of clinical benefit in treating of anemia in pregnancy. But there is no standard dose for this medication during pregnancy.
Aims
To carry out the comparative study to identify more effective treatment regimen and dose of rHuEPO for medication of AP.
Methods
Three groups of anemic pregnant women were enrolled. All groups were stratified according to age, gestational aged and initial mean Hb levels at anemic pregnant women. All of them met the following criteria for inclusion in the study: gestational age above 20 weeks, Hb concentration < 9.5 g/dl, inefficiency of iron therapy alone for at least 4 weeks, and absence of pregnancy complications, or severe systemic diseases. The treatment protocol comprised a combined therapy with rHu-EPO (epoetin-α) subcutaneously and 200 mg iron sulphate orally daily. We used next regimens of rHuEPO dosing: Group 1 (n=18) – 75 IU/kg three times per week (225 IU/kg per week); Group 2 (n=21) – 100 IU/kg three times per week (300 IU/kg per week) and Group 3 (n=16) – 120 IU/kg two times per week (240 IU/kg per week). We evaluated Hb levels weekly beginning before start of rHuEPO therapy during three weeks at all groups of anemic pregnants. The advance of target Hb level (10.5 g/dl for I trimester and 11.0 g/dl for II trimester) in 2 week of rHuEPO therapy we considered as complete response to the medication. Informed consent was obtained from all patients for being included in the study.
Results
Response to the rHuEPO therapy depended on the regimen of dosing (Table).TableComplete response to the rHuEPO therapy of anemic pregnant women depending on the treatment regimen
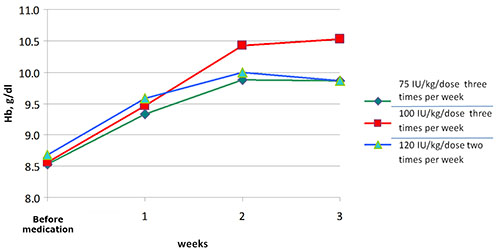
The mean Hb levels, before and in 3 weeks after medication, in group 1 were 8.45±0.28 g/dl and 9.87±0.21 g/dl, in group 2 – 8.17±0.53 g/dl and 10.52±0.28 g/dl, in group 3 – 8.16±0.46 g/dl and 9.87±0.28 g/dl respectively (Fig.). We did not observe any serious adverse effects during the therapy with rHu-EPO.Figure. Comparison of hemoglobin trends in groups of anemic pregnant women who treated with different doses of rHuEPO.
Conclusion
Effectiveness of rHuEPO therapy is dose-dependent. The use of rHu-EPO at dose 100 IU/kg subcutaneously three times per week is more effective treatment regimen for medication of AP.
Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Pregnancy, Treatment
Type: Publication Only
Background
There is evidence that pathogenesis of anemia in pregnancy (AP) is multifactorial. It has not relation to ineffective erythropoiesis, caused by iron or folate deficiency only. Blunted erythropoiesis is one of main AP causes. According to our own data inadequately low production of erythropoietin (EPO) for the degree of the anemia found at more 50% of anemic pregnants. That is why recombinant human erythropoietin (rHuEPO) combined with iron is effective method in the therapy of anemic pregnant women, who had been ineffectively treated with iron alone. Because EPO does not cross the placenta, this protects the fetus from its use during pregnancy. Most recent studies show overall the effect of rHuEpo is of clinical benefit in treating of anemia in pregnancy. But there is no standard dose for this medication during pregnancy.
Aims
To carry out the comparative study to identify more effective treatment regimen and dose of rHuEPO for medication of AP.
Methods
Three groups of anemic pregnant women were enrolled. All groups were stratified according to age, gestational aged and initial mean Hb levels at anemic pregnant women. All of them met the following criteria for inclusion in the study: gestational age above 20 weeks, Hb concentration < 9.5 g/dl, inefficiency of iron therapy alone for at least 4 weeks, and absence of pregnancy complications, or severe systemic diseases. The treatment protocol comprised a combined therapy with rHu-EPO (epoetin-α) subcutaneously and 200 mg iron sulphate orally daily. We used next regimens of rHuEPO dosing: Group 1 (n=18) – 75 IU/kg three times per week (225 IU/kg per week); Group 2 (n=21) – 100 IU/kg three times per week (300 IU/kg per week) and Group 3 (n=16) – 120 IU/kg two times per week (240 IU/kg per week). We evaluated Hb levels weekly beginning before start of rHuEPO therapy during three weeks at all groups of anemic pregnants. The advance of target Hb level (10.5 g/dl for I trimester and 11.0 g/dl for II trimester) in 2 week of rHuEPO therapy we considered as complete response to the medication. Informed consent was obtained from all patients for being included in the study.
Results
Response to the rHuEPO therapy depended on the regimen of dosing (Table).TableComplete response to the rHuEPO therapy of anemic pregnant women depending on the treatment regimen
| Groups of anemic pregnant women | Regimen of dosing, IU/kg/dose | Weekly dose,IU/kg | The number of treated anemic pregnants with complete response to the medication (effectiveness of rHuEPO therapy)n (%) |
| 1 (n=18) | 75three times per week | 225 | 9 (50%) |
| 2 (n=21) | 100three times per week | 300 | 16 (76.5%) |
| 3 (n=16) | 120two times per week | 240 | 7 (43.8%) |
Conclusion
Effectiveness of rHuEPO therapy is dose-dependent. The use of rHu-EPO at dose 100 IU/kg subcutaneously three times per week is more effective treatment regimen for medication of AP.

Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Pregnancy, Treatment
Abstract: PB2124
Type: Publication Only
Background
There is evidence that pathogenesis of anemia in pregnancy (AP) is multifactorial. It has not relation to ineffective erythropoiesis, caused by iron or folate deficiency only. Blunted erythropoiesis is one of main AP causes. According to our own data inadequately low production of erythropoietin (EPO) for the degree of the anemia found at more 50% of anemic pregnants. That is why recombinant human erythropoietin (rHuEPO) combined with iron is effective method in the therapy of anemic pregnant women, who had been ineffectively treated with iron alone. Because EPO does not cross the placenta, this protects the fetus from its use during pregnancy. Most recent studies show overall the effect of rHuEpo is of clinical benefit in treating of anemia in pregnancy. But there is no standard dose for this medication during pregnancy.
Aims
To carry out the comparative study to identify more effective treatment regimen and dose of rHuEPO for medication of AP.
Methods
Three groups of anemic pregnant women were enrolled. All groups were stratified according to age, gestational aged and initial mean Hb levels at anemic pregnant women. All of them met the following criteria for inclusion in the study: gestational age above 20 weeks, Hb concentration < 9.5 g/dl, inefficiency of iron therapy alone for at least 4 weeks, and absence of pregnancy complications, or severe systemic diseases. The treatment protocol comprised a combined therapy with rHu-EPO (epoetin-α) subcutaneously and 200 mg iron sulphate orally daily. We used next regimens of rHuEPO dosing: Group 1 (n=18) – 75 IU/kg three times per week (225 IU/kg per week); Group 2 (n=21) – 100 IU/kg three times per week (300 IU/kg per week) and Group 3 (n=16) – 120 IU/kg two times per week (240 IU/kg per week). We evaluated Hb levels weekly beginning before start of rHuEPO therapy during three weeks at all groups of anemic pregnants. The advance of target Hb level (10.5 g/dl for I trimester and 11.0 g/dl for II trimester) in 2 week of rHuEPO therapy we considered as complete response to the medication. Informed consent was obtained from all patients for being included in the study.
Results
Response to the rHuEPO therapy depended on the regimen of dosing (Table).TableComplete response to the rHuEPO therapy of anemic pregnant women depending on the treatment regimen
The mean Hb levels, before and in 3 weeks after medication, in group 1 were 8.45±0.28 g/dl and 9.87±0.21 g/dl, in group 2 – 8.17±0.53 g/dl and 10.52±0.28 g/dl, in group 3 – 8.16±0.46 g/dl and 9.87±0.28 g/dl respectively (Fig.). We did not observe any serious adverse effects during the therapy with rHu-EPO.Figure. Comparison of hemoglobin trends in groups of anemic pregnant women who treated with different doses of rHuEPO.
Conclusion
Effectiveness of rHuEPO therapy is dose-dependent. The use of rHu-EPO at dose 100 IU/kg subcutaneously three times per week is more effective treatment regimen for medication of AP.

Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Pregnancy, Treatment
Type: Publication Only
Background
There is evidence that pathogenesis of anemia in pregnancy (AP) is multifactorial. It has not relation to ineffective erythropoiesis, caused by iron or folate deficiency only. Blunted erythropoiesis is one of main AP causes. According to our own data inadequately low production of erythropoietin (EPO) for the degree of the anemia found at more 50% of anemic pregnants. That is why recombinant human erythropoietin (rHuEPO) combined with iron is effective method in the therapy of anemic pregnant women, who had been ineffectively treated with iron alone. Because EPO does not cross the placenta, this protects the fetus from its use during pregnancy. Most recent studies show overall the effect of rHuEpo is of clinical benefit in treating of anemia in pregnancy. But there is no standard dose for this medication during pregnancy.
Aims
To carry out the comparative study to identify more effective treatment regimen and dose of rHuEPO for medication of AP.
Methods
Three groups of anemic pregnant women were enrolled. All groups were stratified according to age, gestational aged and initial mean Hb levels at anemic pregnant women. All of them met the following criteria for inclusion in the study: gestational age above 20 weeks, Hb concentration < 9.5 g/dl, inefficiency of iron therapy alone for at least 4 weeks, and absence of pregnancy complications, or severe systemic diseases. The treatment protocol comprised a combined therapy with rHu-EPO (epoetin-α) subcutaneously and 200 mg iron sulphate orally daily. We used next regimens of rHuEPO dosing: Group 1 (n=18) – 75 IU/kg three times per week (225 IU/kg per week); Group 2 (n=21) – 100 IU/kg three times per week (300 IU/kg per week) and Group 3 (n=16) – 120 IU/kg two times per week (240 IU/kg per week). We evaluated Hb levels weekly beginning before start of rHuEPO therapy during three weeks at all groups of anemic pregnants. The advance of target Hb level (10.5 g/dl for I trimester and 11.0 g/dl for II trimester) in 2 week of rHuEPO therapy we considered as complete response to the medication. Informed consent was obtained from all patients for being included in the study.
Results
Response to the rHuEPO therapy depended on the regimen of dosing (Table).TableComplete response to the rHuEPO therapy of anemic pregnant women depending on the treatment regimen
| Groups of anemic pregnant women | Regimen of dosing, IU/kg/dose | Weekly dose,IU/kg | The number of treated anemic pregnants with complete response to the medication (effectiveness of rHuEPO therapy)n (%) |
| 1 (n=18) | 75three times per week | 225 | 9 (50%) |
| 2 (n=21) | 100three times per week | 300 | 16 (76.5%) |
| 3 (n=16) | 120two times per week | 240 | 7 (43.8%) |
Conclusion
Effectiveness of rHuEPO therapy is dose-dependent. The use of rHu-EPO at dose 100 IU/kg subcutaneously three times per week is more effective treatment regimen for medication of AP.

Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Pregnancy, Treatment
{{ help_message }}
{{filter}}


