DISEASE CHARACTERIZATION AND TREATMENT PATTERNS OF PATIENTS WITH MYELOFIBROSIS: ANALYSIS OF US CLAIMS DATABASES
(Abstract release date: 05/19/16)
EHA Library. Mehra M. 06/09/16; 134919; PB2019

Maneesha Mehra
Contributions
Contributions
Abstract
Abstract: PB2019
Type: Publication Only
Background
Myelofibrosis (MF), a rare myeloproliferative neoplasm, is characterized by clonal myeloproliferation, ineffective erythropoiesis, bone marrow stromal changes, hepatosplenic extramedullary hematopoiesis and aberrant cytokine expression. Patients (pts) present with splenomegaly, constitutional symptoms, moderate to severe anemia, thrombocytopenia and leukocytosis. MF has a profound negative impact on survival and quality of life. Presentation may be primary (PMF) or MF transformation from essential thrombocythemia (ET) or polycythemia vera (PV), or may be secondary from diseases such as myelodysplastic syndrome, leukemia, and lymphoma (Other MF). Management options include allogeneic stem cell transplantation, hydroxyurea, interferon alpha, alkylating agents, splenectomy, splenic radiotherapy, and the JAK1/2 inhibitor ruxolitinib.
Aims
Characterize disease and treatment patterns in pts with MF using 2 US health insurance claims databases.
Methods
The Truven Marketscan (Commercial Claims and Encounters and Truven Medicare) database was retrospectively analyzed to identify pts with MF diagnosed between 2006 and 2015 using ICD-9 codes 238.76 and 289.83. Pts aged ≥ 18 years with ≥ 1 month of history prior to diagnosis were included. Pts were categorized as PMF, post-PV/ET MF, or Other MF based on earliest MF diagnosis code. Demographic characteristics, constitutional symptoms, platelet counts, and treatment patterns were summarized. Treatment regimens were analyzed according to the following hierarchy (highest to lowest; “± O” referring to combination with any other treatment listed lower in the hierarchy): ruxolitinib ± O, hydroxyurea ± O, chemotherapy ± O, stem cell transplant ± O, splenectomy ± O, radiation ± O, best supportive care (BSC) ± O, and steroids only. Sub-analysis of ruxolitinib uptake post launch (in 2011) will be presented.
Results
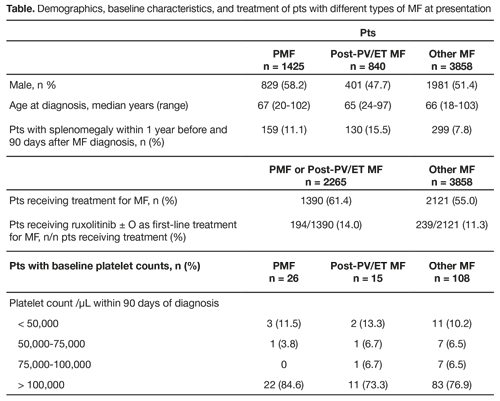
We identified 6795 pts with MF diagnosis between 2006 and 2015. Based on the exclusion criteria, 6123 pts were included; 3211 (52.4%) were male, and median age at diagnosis was 66 years. A total of 1425 (23.3%), 840 (13.7%), and 3858 (63.0%) pts had PMF, post-PV/ET MF, and Other MF, respectively. Prevalence of splenomegaly at baseline was 11.1% in PMF, 15.5% in post-PV/ET MF, and 7.8% in Other MF. Platelet counts at baseline (± 90 days of index MF diagnosis) were available for 149 (2.4%) pts; mean and median platelet levels were 270,323/μL and 218,000/μL, respectively; 16 (10.7%) pts had platelet counts of < 50,000/μL and 116 (77.9%) had platelet counts of > 100,000/μL (Table). Overall 3502/6123 pts (57.2%) received ≥ 1 regimens for MF. Mainstays of initial treatment included hydroxyurea ± O (964 treated pts; 27.5%), and BSC ± O (753; 21.5%). Only 425/3502 treated pts (12.1%) received ruxolitinib ± O as first-line treatment; by category, pts receiving first-line ruxolitinib ± O included 194/1390 treated pts (14.0%) with PMF or post-PV/ET MF and 231/2112 treated pts (10.9%) with Other MF. Hydroxyurea ± O was the most common first-line regimen for pts with PMF or post-PV/ET MF (463/1390; 33.3%); BSC ± O (517/2112; 24.5%) and steroids (615/2112; 29.1%) were the most common first-line regimens for pts with Other MF.
Conclusion
MF was typically diagnosed in pts aged ≥ 65 years, and was associated with splenomegaly or thrombocytopenia at baseline in a minority of pts. In the present database analysis, ~55-60% pts received treatment. Although ruxolitinib was approved in the US in 2011 for treatment of high- or intermediate-risk PMF or post-PV/ET MF, there is limited information in the database on its clinical use as a first-line treatment.
Session topic: E-poster
Keyword(s): Myelofibrosis, Ruxolitinib, Thrombocytopenia
Type: Publication Only
Background
Myelofibrosis (MF), a rare myeloproliferative neoplasm, is characterized by clonal myeloproliferation, ineffective erythropoiesis, bone marrow stromal changes, hepatosplenic extramedullary hematopoiesis and aberrant cytokine expression. Patients (pts) present with splenomegaly, constitutional symptoms, moderate to severe anemia, thrombocytopenia and leukocytosis. MF has a profound negative impact on survival and quality of life. Presentation may be primary (PMF) or MF transformation from essential thrombocythemia (ET) or polycythemia vera (PV), or may be secondary from diseases such as myelodysplastic syndrome, leukemia, and lymphoma (Other MF). Management options include allogeneic stem cell transplantation, hydroxyurea, interferon alpha, alkylating agents, splenectomy, splenic radiotherapy, and the JAK1/2 inhibitor ruxolitinib.
Aims
Characterize disease and treatment patterns in pts with MF using 2 US health insurance claims databases.
Methods
The Truven Marketscan (Commercial Claims and Encounters and Truven Medicare) database was retrospectively analyzed to identify pts with MF diagnosed between 2006 and 2015 using ICD-9 codes 238.76 and 289.83. Pts aged ≥ 18 years with ≥ 1 month of history prior to diagnosis were included. Pts were categorized as PMF, post-PV/ET MF, or Other MF based on earliest MF diagnosis code. Demographic characteristics, constitutional symptoms, platelet counts, and treatment patterns were summarized. Treatment regimens were analyzed according to the following hierarchy (highest to lowest; “± O” referring to combination with any other treatment listed lower in the hierarchy): ruxolitinib ± O, hydroxyurea ± O, chemotherapy ± O, stem cell transplant ± O, splenectomy ± O, radiation ± O, best supportive care (BSC) ± O, and steroids only. Sub-analysis of ruxolitinib uptake post launch (in 2011) will be presented.
Results
We identified 6795 pts with MF diagnosis between 2006 and 2015. Based on the exclusion criteria, 6123 pts were included; 3211 (52.4%) were male, and median age at diagnosis was 66 years. A total of 1425 (23.3%), 840 (13.7%), and 3858 (63.0%) pts had PMF, post-PV/ET MF, and Other MF, respectively. Prevalence of splenomegaly at baseline was 11.1% in PMF, 15.5% in post-PV/ET MF, and 7.8% in Other MF. Platelet counts at baseline (± 90 days of index MF diagnosis) were available for 149 (2.4%) pts; mean and median platelet levels were 270,323/μL and 218,000/μL, respectively; 16 (10.7%) pts had platelet counts of < 50,000/μL and 116 (77.9%) had platelet counts of > 100,000/μL (Table). Overall 3502/6123 pts (57.2%) received ≥ 1 regimens for MF. Mainstays of initial treatment included hydroxyurea ± O (964 treated pts; 27.5%), and BSC ± O (753; 21.5%). Only 425/3502 treated pts (12.1%) received ruxolitinib ± O as first-line treatment; by category, pts receiving first-line ruxolitinib ± O included 194/1390 treated pts (14.0%) with PMF or post-PV/ET MF and 231/2112 treated pts (10.9%) with Other MF. Hydroxyurea ± O was the most common first-line regimen for pts with PMF or post-PV/ET MF (463/1390; 33.3%); BSC ± O (517/2112; 24.5%) and steroids (615/2112; 29.1%) were the most common first-line regimens for pts with Other MF.
Conclusion
MF was typically diagnosed in pts aged ≥ 65 years, and was associated with splenomegaly or thrombocytopenia at baseline in a minority of pts. In the present database analysis, ~55-60% pts received treatment. Although ruxolitinib was approved in the US in 2011 for treatment of high- or intermediate-risk PMF or post-PV/ET MF, there is limited information in the database on its clinical use as a first-line treatment.

Session topic: E-poster
Keyword(s): Myelofibrosis, Ruxolitinib, Thrombocytopenia
Abstract: PB2019
Type: Publication Only
Background
Myelofibrosis (MF), a rare myeloproliferative neoplasm, is characterized by clonal myeloproliferation, ineffective erythropoiesis, bone marrow stromal changes, hepatosplenic extramedullary hematopoiesis and aberrant cytokine expression. Patients (pts) present with splenomegaly, constitutional symptoms, moderate to severe anemia, thrombocytopenia and leukocytosis. MF has a profound negative impact on survival and quality of life. Presentation may be primary (PMF) or MF transformation from essential thrombocythemia (ET) or polycythemia vera (PV), or may be secondary from diseases such as myelodysplastic syndrome, leukemia, and lymphoma (Other MF). Management options include allogeneic stem cell transplantation, hydroxyurea, interferon alpha, alkylating agents, splenectomy, splenic radiotherapy, and the JAK1/2 inhibitor ruxolitinib.
Aims
Characterize disease and treatment patterns in pts with MF using 2 US health insurance claims databases.
Methods
The Truven Marketscan (Commercial Claims and Encounters and Truven Medicare) database was retrospectively analyzed to identify pts with MF diagnosed between 2006 and 2015 using ICD-9 codes 238.76 and 289.83. Pts aged ≥ 18 years with ≥ 1 month of history prior to diagnosis were included. Pts were categorized as PMF, post-PV/ET MF, or Other MF based on earliest MF diagnosis code. Demographic characteristics, constitutional symptoms, platelet counts, and treatment patterns were summarized. Treatment regimens were analyzed according to the following hierarchy (highest to lowest; “± O” referring to combination with any other treatment listed lower in the hierarchy): ruxolitinib ± O, hydroxyurea ± O, chemotherapy ± O, stem cell transplant ± O, splenectomy ± O, radiation ± O, best supportive care (BSC) ± O, and steroids only. Sub-analysis of ruxolitinib uptake post launch (in 2011) will be presented.
Results
We identified 6795 pts with MF diagnosis between 2006 and 2015. Based on the exclusion criteria, 6123 pts were included; 3211 (52.4%) were male, and median age at diagnosis was 66 years. A total of 1425 (23.3%), 840 (13.7%), and 3858 (63.0%) pts had PMF, post-PV/ET MF, and Other MF, respectively. Prevalence of splenomegaly at baseline was 11.1% in PMF, 15.5% in post-PV/ET MF, and 7.8% in Other MF. Platelet counts at baseline (± 90 days of index MF diagnosis) were available for 149 (2.4%) pts; mean and median platelet levels were 270,323/μL and 218,000/μL, respectively; 16 (10.7%) pts had platelet counts of < 50,000/μL and 116 (77.9%) had platelet counts of > 100,000/μL (Table). Overall 3502/6123 pts (57.2%) received ≥ 1 regimens for MF. Mainstays of initial treatment included hydroxyurea ± O (964 treated pts; 27.5%), and BSC ± O (753; 21.5%). Only 425/3502 treated pts (12.1%) received ruxolitinib ± O as first-line treatment; by category, pts receiving first-line ruxolitinib ± O included 194/1390 treated pts (14.0%) with PMF or post-PV/ET MF and 231/2112 treated pts (10.9%) with Other MF. Hydroxyurea ± O was the most common first-line regimen for pts with PMF or post-PV/ET MF (463/1390; 33.3%); BSC ± O (517/2112; 24.5%) and steroids (615/2112; 29.1%) were the most common first-line regimens for pts with Other MF.
Conclusion
MF was typically diagnosed in pts aged ≥ 65 years, and was associated with splenomegaly or thrombocytopenia at baseline in a minority of pts. In the present database analysis, ~55-60% pts received treatment. Although ruxolitinib was approved in the US in 2011 for treatment of high- or intermediate-risk PMF or post-PV/ET MF, there is limited information in the database on its clinical use as a first-line treatment.

Session topic: E-poster
Keyword(s): Myelofibrosis, Ruxolitinib, Thrombocytopenia
Type: Publication Only
Background
Myelofibrosis (MF), a rare myeloproliferative neoplasm, is characterized by clonal myeloproliferation, ineffective erythropoiesis, bone marrow stromal changes, hepatosplenic extramedullary hematopoiesis and aberrant cytokine expression. Patients (pts) present with splenomegaly, constitutional symptoms, moderate to severe anemia, thrombocytopenia and leukocytosis. MF has a profound negative impact on survival and quality of life. Presentation may be primary (PMF) or MF transformation from essential thrombocythemia (ET) or polycythemia vera (PV), or may be secondary from diseases such as myelodysplastic syndrome, leukemia, and lymphoma (Other MF). Management options include allogeneic stem cell transplantation, hydroxyurea, interferon alpha, alkylating agents, splenectomy, splenic radiotherapy, and the JAK1/2 inhibitor ruxolitinib.
Aims
Characterize disease and treatment patterns in pts with MF using 2 US health insurance claims databases.
Methods
The Truven Marketscan (Commercial Claims and Encounters and Truven Medicare) database was retrospectively analyzed to identify pts with MF diagnosed between 2006 and 2015 using ICD-9 codes 238.76 and 289.83. Pts aged ≥ 18 years with ≥ 1 month of history prior to diagnosis were included. Pts were categorized as PMF, post-PV/ET MF, or Other MF based on earliest MF diagnosis code. Demographic characteristics, constitutional symptoms, platelet counts, and treatment patterns were summarized. Treatment regimens were analyzed according to the following hierarchy (highest to lowest; “± O” referring to combination with any other treatment listed lower in the hierarchy): ruxolitinib ± O, hydroxyurea ± O, chemotherapy ± O, stem cell transplant ± O, splenectomy ± O, radiation ± O, best supportive care (BSC) ± O, and steroids only. Sub-analysis of ruxolitinib uptake post launch (in 2011) will be presented.
Results
We identified 6795 pts with MF diagnosis between 2006 and 2015. Based on the exclusion criteria, 6123 pts were included; 3211 (52.4%) were male, and median age at diagnosis was 66 years. A total of 1425 (23.3%), 840 (13.7%), and 3858 (63.0%) pts had PMF, post-PV/ET MF, and Other MF, respectively. Prevalence of splenomegaly at baseline was 11.1% in PMF, 15.5% in post-PV/ET MF, and 7.8% in Other MF. Platelet counts at baseline (± 90 days of index MF diagnosis) were available for 149 (2.4%) pts; mean and median platelet levels were 270,323/μL and 218,000/μL, respectively; 16 (10.7%) pts had platelet counts of < 50,000/μL and 116 (77.9%) had platelet counts of > 100,000/μL (Table). Overall 3502/6123 pts (57.2%) received ≥ 1 regimens for MF. Mainstays of initial treatment included hydroxyurea ± O (964 treated pts; 27.5%), and BSC ± O (753; 21.5%). Only 425/3502 treated pts (12.1%) received ruxolitinib ± O as first-line treatment; by category, pts receiving first-line ruxolitinib ± O included 194/1390 treated pts (14.0%) with PMF or post-PV/ET MF and 231/2112 treated pts (10.9%) with Other MF. Hydroxyurea ± O was the most common first-line regimen for pts with PMF or post-PV/ET MF (463/1390; 33.3%); BSC ± O (517/2112; 24.5%) and steroids (615/2112; 29.1%) were the most common first-line regimens for pts with Other MF.
Conclusion
MF was typically diagnosed in pts aged ≥ 65 years, and was associated with splenomegaly or thrombocytopenia at baseline in a minority of pts. In the present database analysis, ~55-60% pts received treatment. Although ruxolitinib was approved in the US in 2011 for treatment of high- or intermediate-risk PMF or post-PV/ET MF, there is limited information in the database on its clinical use as a first-line treatment.

Session topic: E-poster
Keyword(s): Myelofibrosis, Ruxolitinib, Thrombocytopenia
{{ help_message }}
{{filter}}


