THROMBOTIC MICROANGIOPATHY ASSOCIATED WITH BORTEZOMIB TREATMENT IN MULTIPLE MYELOMA
(Abstract release date: 05/19/16)
EHA Library. Pedraza A. 06/09/16; 134903; PB2003

Dr. Alexandra Pedraza
Contributions
Contributions
Abstract
Abstract: PB2003
Type: Publication Only
Background
Thrombotic microangiopathy (TMA) is a very diverse medical condition defined by the presence of microangiopathic hemolytic anemia (MAHA), thrombocytopenia and microvascular thrombosis, being characteristic of the MAHA schistocytes in peripheral blood. From an etiopatogenics point of view, it can be classified as primary, when there is clear evidence of a disorder caused by TMA, as the TTP and HUS, and secondary, when the TMA is a symptom of underlying conditions such as tumor processes, pregnancy, HIV infection, bone marrow transplant and exposure to certain drugs. Today, drug induced TMA is clearly recognized and it has been reported that at least 22 drugs involved in this process, including various chemotherapeutic agents (cyclosporine, mitomycin C, gemcitabine, bevacizumab, ect) and other substances such as vaccines, herbal products and homeopathic drugs.
Aims
We show the sixth case reported to date of bortezomib-induced thrombotic microangiopathy in MM.
Methods
52 year-old woman diagnosed with multiple myeloma stage IIIA IgG Lambda ISS II with anemia and without thrombocytopenia, creatinine 0.84 mg/dL and glomerular filtration rate of 81 mL/min, discreet elevation of LDH and Beta2 microglobulin, monoclonal component of 2.2 g/dL IgG lambda and urine 6390 mg/dl, bone lesions in dorsolumbar vertebrae and medullogram with infiltration for plasmatic cells monoclonales of 25-35 %. She starts first-line treatment with bortezomib, lenalidomide and dexamethasone. The eleventh day of treatment presents sudden increase of creatinine and a decrease in the filtration rate, oliguria, and the progressive decrease of platelets. In addition, hypertransaminasemia with hyperbilirubinemia and increased PCR, gradual rise in LDH and decreased haptoglobin documented. In the peripheral blood smear the platelet count was verified and frequent presence of schistocytes was reported. Within the analytical study, not immune anemia is found with a negative Coombs test, normal coagulation tests, serology, Shiga toxin in feces and negative pregnancy test, ADAMTS13 activity of 33% and negative ADAMS13 antibodies, and complement levels were normal. The treatment was started with plasmapheresis until all 12 sessions were completed with progressive improvement. After 5 additional cycles of chemotherapy with dexamethasone and lenaidomida without bortezomib, and an autologous stem cell transplant, she reached very good partial response.
Results
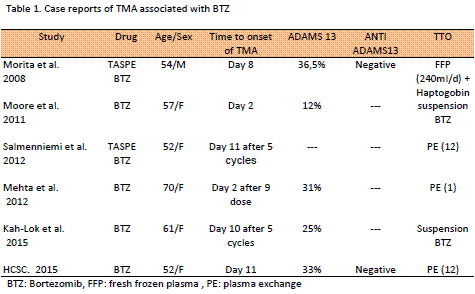
So far, 5 cases of post TMA bortezomib have been described in the literature (Table 1).The acute immunological reaction and the drug toxicity are the two recognized hypothesis that explain the development of thrombotic microangiopathy secondary to drugs. The variavility in the time of the beginning of the TMA in five cases, ADAMS13's different values and the absence of antibodies in one of them, suggests that not only the immune mechanism is involved in his etiopatogenia. Nevertheless, other drugs such as ticlopidine, produce MAT by a clearly immune mechanism unleashing a severe decrease of the activity of ADAMS13 by the production of antibodies anti ADAMS13. Though, the immunomodulatory capacity of the bortezomib inside the bone marrow microenvironment was demonstrated, still it is not known if such a condition could have any relation with the development of MAT; of fact, a case of microangiopathy successfully treated with bortezomib has been reported, arguing the blockage of plasma cells and B-lymphocytes autoreativos. On the other hand, other medicines like the monoclonal antibodies anti VEGF (ej. bevacizumab) that alter directly the angiogenesis have been associated with TMA. Bortezomib in addition from blocking the action of the proteasome, also suppresses the production and secretion of VEGF, which could be related to an altered angiogenesis and endothelial damage characteristic of TMA. For the treatment of MAT diverse empirical therapies were used, without restarting the bortezomib in any of five cases and the clinical being solved in all of them.
Conclusion
TMA must be recognized as a potentially serious secondary side effect to its administration of bortezmib in patients who develop MAHA, thrombocytopenia and acute renal failure.
Session topic: E-poster
Keyword(s): Bortezomib, Multiple myeloma, Thrombotic microangiopathy
Type: Publication Only
Background
Thrombotic microangiopathy (TMA) is a very diverse medical condition defined by the presence of microangiopathic hemolytic anemia (MAHA), thrombocytopenia and microvascular thrombosis, being characteristic of the MAHA schistocytes in peripheral blood. From an etiopatogenics point of view, it can be classified as primary, when there is clear evidence of a disorder caused by TMA, as the TTP and HUS, and secondary, when the TMA is a symptom of underlying conditions such as tumor processes, pregnancy, HIV infection, bone marrow transplant and exposure to certain drugs. Today, drug induced TMA is clearly recognized and it has been reported that at least 22 drugs involved in this process, including various chemotherapeutic agents (cyclosporine, mitomycin C, gemcitabine, bevacizumab, ect) and other substances such as vaccines, herbal products and homeopathic drugs.
Aims
We show the sixth case reported to date of bortezomib-induced thrombotic microangiopathy in MM.
Methods
52 year-old woman diagnosed with multiple myeloma stage IIIA IgG Lambda ISS II with anemia and without thrombocytopenia, creatinine 0.84 mg/dL and glomerular filtration rate of 81 mL/min, discreet elevation of LDH and Beta2 microglobulin, monoclonal component of 2.2 g/dL IgG lambda and urine 6390 mg/dl, bone lesions in dorsolumbar vertebrae and medullogram with infiltration for plasmatic cells monoclonales of 25-35 %. She starts first-line treatment with bortezomib, lenalidomide and dexamethasone. The eleventh day of treatment presents sudden increase of creatinine and a decrease in the filtration rate, oliguria, and the progressive decrease of platelets. In addition, hypertransaminasemia with hyperbilirubinemia and increased PCR, gradual rise in LDH and decreased haptoglobin documented. In the peripheral blood smear the platelet count was verified and frequent presence of schistocytes was reported. Within the analytical study, not immune anemia is found with a negative Coombs test, normal coagulation tests, serology, Shiga toxin in feces and negative pregnancy test, ADAMTS13 activity of 33% and negative ADAMS13 antibodies, and complement levels were normal. The treatment was started with plasmapheresis until all 12 sessions were completed with progressive improvement. After 5 additional cycles of chemotherapy with dexamethasone and lenaidomida without bortezomib, and an autologous stem cell transplant, she reached very good partial response.
Results
So far, 5 cases of post TMA bortezomib have been described in the literature (Table 1).The acute immunological reaction and the drug toxicity are the two recognized hypothesis that explain the development of thrombotic microangiopathy secondary to drugs. The variavility in the time of the beginning of the TMA in five cases, ADAMS13's different values and the absence of antibodies in one of them, suggests that not only the immune mechanism is involved in his etiopatogenia. Nevertheless, other drugs such as ticlopidine, produce MAT by a clearly immune mechanism unleashing a severe decrease of the activity of ADAMS13 by the production of antibodies anti ADAMS13. Though, the immunomodulatory capacity of the bortezomib inside the bone marrow microenvironment was demonstrated, still it is not known if such a condition could have any relation with the development of MAT; of fact, a case of microangiopathy successfully treated with bortezomib has been reported, arguing the blockage of plasma cells and B-lymphocytes autoreativos. On the other hand, other medicines like the monoclonal antibodies anti VEGF (ej. bevacizumab) that alter directly the angiogenesis have been associated with TMA. Bortezomib in addition from blocking the action of the proteasome, also suppresses the production and secretion of VEGF, which could be related to an altered angiogenesis and endothelial damage characteristic of TMA. For the treatment of MAT diverse empirical therapies were used, without restarting the bortezomib in any of five cases and the clinical being solved in all of them.
Conclusion
TMA must be recognized as a potentially serious secondary side effect to its administration of bortezmib in patients who develop MAHA, thrombocytopenia and acute renal failure.

Session topic: E-poster
Keyword(s): Bortezomib, Multiple myeloma, Thrombotic microangiopathy
Abstract: PB2003
Type: Publication Only
Background
Thrombotic microangiopathy (TMA) is a very diverse medical condition defined by the presence of microangiopathic hemolytic anemia (MAHA), thrombocytopenia and microvascular thrombosis, being characteristic of the MAHA schistocytes in peripheral blood. From an etiopatogenics point of view, it can be classified as primary, when there is clear evidence of a disorder caused by TMA, as the TTP and HUS, and secondary, when the TMA is a symptom of underlying conditions such as tumor processes, pregnancy, HIV infection, bone marrow transplant and exposure to certain drugs. Today, drug induced TMA is clearly recognized and it has been reported that at least 22 drugs involved in this process, including various chemotherapeutic agents (cyclosporine, mitomycin C, gemcitabine, bevacizumab, ect) and other substances such as vaccines, herbal products and homeopathic drugs.
Aims
We show the sixth case reported to date of bortezomib-induced thrombotic microangiopathy in MM.
Methods
52 year-old woman diagnosed with multiple myeloma stage IIIA IgG Lambda ISS II with anemia and without thrombocytopenia, creatinine 0.84 mg/dL and glomerular filtration rate of 81 mL/min, discreet elevation of LDH and Beta2 microglobulin, monoclonal component of 2.2 g/dL IgG lambda and urine 6390 mg/dl, bone lesions in dorsolumbar vertebrae and medullogram with infiltration for plasmatic cells monoclonales of 25-35 %. She starts first-line treatment with bortezomib, lenalidomide and dexamethasone. The eleventh day of treatment presents sudden increase of creatinine and a decrease in the filtration rate, oliguria, and the progressive decrease of platelets. In addition, hypertransaminasemia with hyperbilirubinemia and increased PCR, gradual rise in LDH and decreased haptoglobin documented. In the peripheral blood smear the platelet count was verified and frequent presence of schistocytes was reported. Within the analytical study, not immune anemia is found with a negative Coombs test, normal coagulation tests, serology, Shiga toxin in feces and negative pregnancy test, ADAMTS13 activity of 33% and negative ADAMS13 antibodies, and complement levels were normal. The treatment was started with plasmapheresis until all 12 sessions were completed with progressive improvement. After 5 additional cycles of chemotherapy with dexamethasone and lenaidomida without bortezomib, and an autologous stem cell transplant, she reached very good partial response.
Results
So far, 5 cases of post TMA bortezomib have been described in the literature (Table 1).The acute immunological reaction and the drug toxicity are the two recognized hypothesis that explain the development of thrombotic microangiopathy secondary to drugs. The variavility in the time of the beginning of the TMA in five cases, ADAMS13's different values and the absence of antibodies in one of them, suggests that not only the immune mechanism is involved in his etiopatogenia. Nevertheless, other drugs such as ticlopidine, produce MAT by a clearly immune mechanism unleashing a severe decrease of the activity of ADAMS13 by the production of antibodies anti ADAMS13. Though, the immunomodulatory capacity of the bortezomib inside the bone marrow microenvironment was demonstrated, still it is not known if such a condition could have any relation with the development of MAT; of fact, a case of microangiopathy successfully treated with bortezomib has been reported, arguing the blockage of plasma cells and B-lymphocytes autoreativos. On the other hand, other medicines like the monoclonal antibodies anti VEGF (ej. bevacizumab) that alter directly the angiogenesis have been associated with TMA. Bortezomib in addition from blocking the action of the proteasome, also suppresses the production and secretion of VEGF, which could be related to an altered angiogenesis and endothelial damage characteristic of TMA. For the treatment of MAT diverse empirical therapies were used, without restarting the bortezomib in any of five cases and the clinical being solved in all of them.
Conclusion
TMA must be recognized as a potentially serious secondary side effect to its administration of bortezmib in patients who develop MAHA, thrombocytopenia and acute renal failure.

Session topic: E-poster
Keyword(s): Bortezomib, Multiple myeloma, Thrombotic microangiopathy
Type: Publication Only
Background
Thrombotic microangiopathy (TMA) is a very diverse medical condition defined by the presence of microangiopathic hemolytic anemia (MAHA), thrombocytopenia and microvascular thrombosis, being characteristic of the MAHA schistocytes in peripheral blood. From an etiopatogenics point of view, it can be classified as primary, when there is clear evidence of a disorder caused by TMA, as the TTP and HUS, and secondary, when the TMA is a symptom of underlying conditions such as tumor processes, pregnancy, HIV infection, bone marrow transplant and exposure to certain drugs. Today, drug induced TMA is clearly recognized and it has been reported that at least 22 drugs involved in this process, including various chemotherapeutic agents (cyclosporine, mitomycin C, gemcitabine, bevacizumab, ect) and other substances such as vaccines, herbal products and homeopathic drugs.
Aims
We show the sixth case reported to date of bortezomib-induced thrombotic microangiopathy in MM.
Methods
52 year-old woman diagnosed with multiple myeloma stage IIIA IgG Lambda ISS II with anemia and without thrombocytopenia, creatinine 0.84 mg/dL and glomerular filtration rate of 81 mL/min, discreet elevation of LDH and Beta2 microglobulin, monoclonal component of 2.2 g/dL IgG lambda and urine 6390 mg/dl, bone lesions in dorsolumbar vertebrae and medullogram with infiltration for plasmatic cells monoclonales of 25-35 %. She starts first-line treatment with bortezomib, lenalidomide and dexamethasone. The eleventh day of treatment presents sudden increase of creatinine and a decrease in the filtration rate, oliguria, and the progressive decrease of platelets. In addition, hypertransaminasemia with hyperbilirubinemia and increased PCR, gradual rise in LDH and decreased haptoglobin documented. In the peripheral blood smear the platelet count was verified and frequent presence of schistocytes was reported. Within the analytical study, not immune anemia is found with a negative Coombs test, normal coagulation tests, serology, Shiga toxin in feces and negative pregnancy test, ADAMTS13 activity of 33% and negative ADAMS13 antibodies, and complement levels were normal. The treatment was started with plasmapheresis until all 12 sessions were completed with progressive improvement. After 5 additional cycles of chemotherapy with dexamethasone and lenaidomida without bortezomib, and an autologous stem cell transplant, she reached very good partial response.
Results
So far, 5 cases of post TMA bortezomib have been described in the literature (Table 1).The acute immunological reaction and the drug toxicity are the two recognized hypothesis that explain the development of thrombotic microangiopathy secondary to drugs. The variavility in the time of the beginning of the TMA in five cases, ADAMS13's different values and the absence of antibodies in one of them, suggests that not only the immune mechanism is involved in his etiopatogenia. Nevertheless, other drugs such as ticlopidine, produce MAT by a clearly immune mechanism unleashing a severe decrease of the activity of ADAMS13 by the production of antibodies anti ADAMS13. Though, the immunomodulatory capacity of the bortezomib inside the bone marrow microenvironment was demonstrated, still it is not known if such a condition could have any relation with the development of MAT; of fact, a case of microangiopathy successfully treated with bortezomib has been reported, arguing the blockage of plasma cells and B-lymphocytes autoreativos. On the other hand, other medicines like the monoclonal antibodies anti VEGF (ej. bevacizumab) that alter directly the angiogenesis have been associated with TMA. Bortezomib in addition from blocking the action of the proteasome, also suppresses the production and secretion of VEGF, which could be related to an altered angiogenesis and endothelial damage characteristic of TMA. For the treatment of MAT diverse empirical therapies were used, without restarting the bortezomib in any of five cases and the clinical being solved in all of them.
Conclusion
TMA must be recognized as a potentially serious secondary side effect to its administration of bortezmib in patients who develop MAHA, thrombocytopenia and acute renal failure.

Session topic: E-poster
Keyword(s): Bortezomib, Multiple myeloma, Thrombotic microangiopathy
{{ help_message }}
{{filter}}


