A PHASE 2 TRIAL OF SMALL-DOSE BORTEZOMIB, LENALIDOMIDE, AND DEXAMETHASONE (SVRD) AS CONSOLIDATION/MAINTENANCE THERAPY IN PATIENTS WITH MULTIPLE MYELOMA
(Abstract release date: 05/19/16)
EHA Library. Sato T. 06/09/16; 134875; PB1975

Dr. Tsutomu Sato
Contributions
Contributions
Abstract
Abstract: PB1975
Type: Publication Only
Background
Consolidation (two to four cycles of combination therapies) and maintenance (continuous therapy, usually with single agents, until the time of disease progression) are commonly used in clinical practice after induction therapy for patients with multiple myeloma (MM). There have been many trials to support the use of consolidation/maintenance to maintain the response achieved after autologous hematopoietic stem cell transplantation or conventional treatments and to improve patient survival with single agent or combination therapy. However, no definitive information is available regarding which drug or which combination of drugs is the most favorable for consolidation/maintenance. The combination therapy with bortezomib, lenalidomide and dexamethasone (VRD) is a powerful regimen for relapsed/refractory as well as newly diagnosed MM as an induction therapy. However, severe adverse events (AEs) may become a problem when VRD is introduced without dose-reduction as a consolidation/maintenance therapy.
Aims
The aim of this multicenter, open-label, single-arm, phase 2 study was to determine the efficacy and safety of reduced-dose VRD regimen (small VRD: sVRD) in Japanese patients with MM in the consolidation/maintenance setting.
Methods
Eligible patients were age ≥20 and ≤80 years, with measurable symptomatic MM. Patients must have received at least 1 prior regimen and achieved at least a partial response (PR) by the International Myeloma Working Group (IMWG) Uniform Response Criteria. Patients received subcutaneous bortezomib (1.3 mg/m2 on days 1 and 15), oral lenalidomide (10 mg on days 1-21) and oral dexamethasone (40 mg on days 1, 8, 15 and 22). The course was repeated every 4 weeks for 6 cycles. Patients with at least a PR at the end of cycle 6 could continue sVRD treatment. Patients discontinued therapy if they experienced progressive disease (PD) or unacceptable toxicity, if no more additional benefits could be expected, or if the patient/investigator decided to discontinue therapy for any reason.
Results
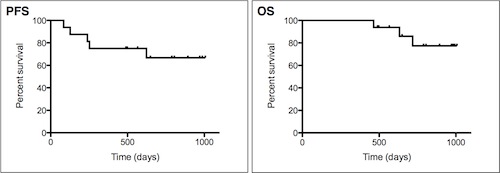
Sixteen patients were enrolled. All patients could complete 6 courses of sVRD treatment. The median duration of sVRD treatment was 8.0 courses (range, 6-28 courses), with 56.3% (n=9) and 25.0% (n=4) undergoing >6 and >12 cycles, respectively. The reasons for treatment discontinuation were completion of 6 courses (n=7, 43.8%), disease progression (n=3, 18.8%), secondary primary malignancies (SPM) of acute lymphoblastic leukemia (ALL) (n=1, 6.3%), AE of grade 2 pneumonia (n=1, 6.3%), or other (patient refusal or physician preference) (n=4, 25.0%). The overall response rate and the complete response (CR) rate were 100% and 43.8%, respectively. In particular, one patient with CR and two patients with very good PR (VGPR) at enrollment achieved stringent CR (sCR) during 6 courses of sVRD. In 9 patients with PR at enrollment, 1 achieved VGPR, but 8 remained in PR. Nevertheless, 2 out of 8 patients with PR after 6 courses of sVRD finally achieved VGPR or sCR after a total of 18 or 24 courses of sVRD, respectively. With a median follow-up time of 29.4 months, the median progression-free survival (PFS) and overall survival (OS) were not reached, while the PFS and OS rates at 2.5 years were 66.6% and 77.3%, respectively. Three patients died and their cause of death was disease progression in all cases. It is noteworthy that the three patients who discontinued sVRD treatment due to PD were the same three patients who died in spite of various post-study therapies. Univariate analysis demonstrated that disease progression as a reason for discontinuation of sVRD had a negative impact on OS. One dose modification of dexamethasone from 40 mg/day to 20 mg/day was required because of grade 2 hypertension after the 3rd course of sVRD. One patient had discontinued all study drugs because of grade 2 pneumonia after 6 courses of sVRD. There were no grade 3 or 4 hematologic or nonhematologic AEs. After enrollment, 2 new hematologic malignancies, i.e., ALL in one patient and myelodysplastic syndrome (MDS) in another patient, were diagnosed.
Conclusion
Our sVRD regimen as a consolidation/maintenance therapy was well-tolerable and highly effective in patients with MM who achieved at least PR after any induction therapy. We conclude that the dosage of bortezomib and lenalidomide in our sVRD regimen may be able to reduce AEs and have preserved efficacy simultaneously in the consolidation/maintenance setting.
Session topic: E-poster
Keyword(s): Consolidation, Maintenance, Multiple myeloma
Type: Publication Only
Background
Consolidation (two to four cycles of combination therapies) and maintenance (continuous therapy, usually with single agents, until the time of disease progression) are commonly used in clinical practice after induction therapy for patients with multiple myeloma (MM). There have been many trials to support the use of consolidation/maintenance to maintain the response achieved after autologous hematopoietic stem cell transplantation or conventional treatments and to improve patient survival with single agent or combination therapy. However, no definitive information is available regarding which drug or which combination of drugs is the most favorable for consolidation/maintenance. The combination therapy with bortezomib, lenalidomide and dexamethasone (VRD) is a powerful regimen for relapsed/refractory as well as newly diagnosed MM as an induction therapy. However, severe adverse events (AEs) may become a problem when VRD is introduced without dose-reduction as a consolidation/maintenance therapy.
Aims
The aim of this multicenter, open-label, single-arm, phase 2 study was to determine the efficacy and safety of reduced-dose VRD regimen (small VRD: sVRD) in Japanese patients with MM in the consolidation/maintenance setting.
Methods
Eligible patients were age ≥20 and ≤80 years, with measurable symptomatic MM. Patients must have received at least 1 prior regimen and achieved at least a partial response (PR) by the International Myeloma Working Group (IMWG) Uniform Response Criteria. Patients received subcutaneous bortezomib (1.3 mg/m2 on days 1 and 15), oral lenalidomide (10 mg on days 1-21) and oral dexamethasone (40 mg on days 1, 8, 15 and 22). The course was repeated every 4 weeks for 6 cycles. Patients with at least a PR at the end of cycle 6 could continue sVRD treatment. Patients discontinued therapy if they experienced progressive disease (PD) or unacceptable toxicity, if no more additional benefits could be expected, or if the patient/investigator decided to discontinue therapy for any reason.
Results
Sixteen patients were enrolled. All patients could complete 6 courses of sVRD treatment. The median duration of sVRD treatment was 8.0 courses (range, 6-28 courses), with 56.3% (n=9) and 25.0% (n=4) undergoing >6 and >12 cycles, respectively. The reasons for treatment discontinuation were completion of 6 courses (n=7, 43.8%), disease progression (n=3, 18.8%), secondary primary malignancies (SPM) of acute lymphoblastic leukemia (ALL) (n=1, 6.3%), AE of grade 2 pneumonia (n=1, 6.3%), or other (patient refusal or physician preference) (n=4, 25.0%). The overall response rate and the complete response (CR) rate were 100% and 43.8%, respectively. In particular, one patient with CR and two patients with very good PR (VGPR) at enrollment achieved stringent CR (sCR) during 6 courses of sVRD. In 9 patients with PR at enrollment, 1 achieved VGPR, but 8 remained in PR. Nevertheless, 2 out of 8 patients with PR after 6 courses of sVRD finally achieved VGPR or sCR after a total of 18 or 24 courses of sVRD, respectively. With a median follow-up time of 29.4 months, the median progression-free survival (PFS) and overall survival (OS) were not reached, while the PFS and OS rates at 2.5 years were 66.6% and 77.3%, respectively. Three patients died and their cause of death was disease progression in all cases. It is noteworthy that the three patients who discontinued sVRD treatment due to PD were the same three patients who died in spite of various post-study therapies. Univariate analysis demonstrated that disease progression as a reason for discontinuation of sVRD had a negative impact on OS. One dose modification of dexamethasone from 40 mg/day to 20 mg/day was required because of grade 2 hypertension after the 3rd course of sVRD. One patient had discontinued all study drugs because of grade 2 pneumonia after 6 courses of sVRD. There were no grade 3 or 4 hematologic or nonhematologic AEs. After enrollment, 2 new hematologic malignancies, i.e., ALL in one patient and myelodysplastic syndrome (MDS) in another patient, were diagnosed.
Conclusion
Our sVRD regimen as a consolidation/maintenance therapy was well-tolerable and highly effective in patients with MM who achieved at least PR after any induction therapy. We conclude that the dosage of bortezomib and lenalidomide in our sVRD regimen may be able to reduce AEs and have preserved efficacy simultaneously in the consolidation/maintenance setting.

Session topic: E-poster
Keyword(s): Consolidation, Maintenance, Multiple myeloma
Abstract: PB1975
Type: Publication Only
Background
Consolidation (two to four cycles of combination therapies) and maintenance (continuous therapy, usually with single agents, until the time of disease progression) are commonly used in clinical practice after induction therapy for patients with multiple myeloma (MM). There have been many trials to support the use of consolidation/maintenance to maintain the response achieved after autologous hematopoietic stem cell transplantation or conventional treatments and to improve patient survival with single agent or combination therapy. However, no definitive information is available regarding which drug or which combination of drugs is the most favorable for consolidation/maintenance. The combination therapy with bortezomib, lenalidomide and dexamethasone (VRD) is a powerful regimen for relapsed/refractory as well as newly diagnosed MM as an induction therapy. However, severe adverse events (AEs) may become a problem when VRD is introduced without dose-reduction as a consolidation/maintenance therapy.
Aims
The aim of this multicenter, open-label, single-arm, phase 2 study was to determine the efficacy and safety of reduced-dose VRD regimen (small VRD: sVRD) in Japanese patients with MM in the consolidation/maintenance setting.
Methods
Eligible patients were age ≥20 and ≤80 years, with measurable symptomatic MM. Patients must have received at least 1 prior regimen and achieved at least a partial response (PR) by the International Myeloma Working Group (IMWG) Uniform Response Criteria. Patients received subcutaneous bortezomib (1.3 mg/m2 on days 1 and 15), oral lenalidomide (10 mg on days 1-21) and oral dexamethasone (40 mg on days 1, 8, 15 and 22). The course was repeated every 4 weeks for 6 cycles. Patients with at least a PR at the end of cycle 6 could continue sVRD treatment. Patients discontinued therapy if they experienced progressive disease (PD) or unacceptable toxicity, if no more additional benefits could be expected, or if the patient/investigator decided to discontinue therapy for any reason.
Results
Sixteen patients were enrolled. All patients could complete 6 courses of sVRD treatment. The median duration of sVRD treatment was 8.0 courses (range, 6-28 courses), with 56.3% (n=9) and 25.0% (n=4) undergoing >6 and >12 cycles, respectively. The reasons for treatment discontinuation were completion of 6 courses (n=7, 43.8%), disease progression (n=3, 18.8%), secondary primary malignancies (SPM) of acute lymphoblastic leukemia (ALL) (n=1, 6.3%), AE of grade 2 pneumonia (n=1, 6.3%), or other (patient refusal or physician preference) (n=4, 25.0%). The overall response rate and the complete response (CR) rate were 100% and 43.8%, respectively. In particular, one patient with CR and two patients with very good PR (VGPR) at enrollment achieved stringent CR (sCR) during 6 courses of sVRD. In 9 patients with PR at enrollment, 1 achieved VGPR, but 8 remained in PR. Nevertheless, 2 out of 8 patients with PR after 6 courses of sVRD finally achieved VGPR or sCR after a total of 18 or 24 courses of sVRD, respectively. With a median follow-up time of 29.4 months, the median progression-free survival (PFS) and overall survival (OS) were not reached, while the PFS and OS rates at 2.5 years were 66.6% and 77.3%, respectively. Three patients died and their cause of death was disease progression in all cases. It is noteworthy that the three patients who discontinued sVRD treatment due to PD were the same three patients who died in spite of various post-study therapies. Univariate analysis demonstrated that disease progression as a reason for discontinuation of sVRD had a negative impact on OS. One dose modification of dexamethasone from 40 mg/day to 20 mg/day was required because of grade 2 hypertension after the 3rd course of sVRD. One patient had discontinued all study drugs because of grade 2 pneumonia after 6 courses of sVRD. There were no grade 3 or 4 hematologic or nonhematologic AEs. After enrollment, 2 new hematologic malignancies, i.e., ALL in one patient and myelodysplastic syndrome (MDS) in another patient, were diagnosed.
Conclusion
Our sVRD regimen as a consolidation/maintenance therapy was well-tolerable and highly effective in patients with MM who achieved at least PR after any induction therapy. We conclude that the dosage of bortezomib and lenalidomide in our sVRD regimen may be able to reduce AEs and have preserved efficacy simultaneously in the consolidation/maintenance setting.

Session topic: E-poster
Keyword(s): Consolidation, Maintenance, Multiple myeloma
Type: Publication Only
Background
Consolidation (two to four cycles of combination therapies) and maintenance (continuous therapy, usually with single agents, until the time of disease progression) are commonly used in clinical practice after induction therapy for patients with multiple myeloma (MM). There have been many trials to support the use of consolidation/maintenance to maintain the response achieved after autologous hematopoietic stem cell transplantation or conventional treatments and to improve patient survival with single agent or combination therapy. However, no definitive information is available regarding which drug or which combination of drugs is the most favorable for consolidation/maintenance. The combination therapy with bortezomib, lenalidomide and dexamethasone (VRD) is a powerful regimen for relapsed/refractory as well as newly diagnosed MM as an induction therapy. However, severe adverse events (AEs) may become a problem when VRD is introduced without dose-reduction as a consolidation/maintenance therapy.
Aims
The aim of this multicenter, open-label, single-arm, phase 2 study was to determine the efficacy and safety of reduced-dose VRD regimen (small VRD: sVRD) in Japanese patients with MM in the consolidation/maintenance setting.
Methods
Eligible patients were age ≥20 and ≤80 years, with measurable symptomatic MM. Patients must have received at least 1 prior regimen and achieved at least a partial response (PR) by the International Myeloma Working Group (IMWG) Uniform Response Criteria. Patients received subcutaneous bortezomib (1.3 mg/m2 on days 1 and 15), oral lenalidomide (10 mg on days 1-21) and oral dexamethasone (40 mg on days 1, 8, 15 and 22). The course was repeated every 4 weeks for 6 cycles. Patients with at least a PR at the end of cycle 6 could continue sVRD treatment. Patients discontinued therapy if they experienced progressive disease (PD) or unacceptable toxicity, if no more additional benefits could be expected, or if the patient/investigator decided to discontinue therapy for any reason.
Results
Sixteen patients were enrolled. All patients could complete 6 courses of sVRD treatment. The median duration of sVRD treatment was 8.0 courses (range, 6-28 courses), with 56.3% (n=9) and 25.0% (n=4) undergoing >6 and >12 cycles, respectively. The reasons for treatment discontinuation were completion of 6 courses (n=7, 43.8%), disease progression (n=3, 18.8%), secondary primary malignancies (SPM) of acute lymphoblastic leukemia (ALL) (n=1, 6.3%), AE of grade 2 pneumonia (n=1, 6.3%), or other (patient refusal or physician preference) (n=4, 25.0%). The overall response rate and the complete response (CR) rate were 100% and 43.8%, respectively. In particular, one patient with CR and two patients with very good PR (VGPR) at enrollment achieved stringent CR (sCR) during 6 courses of sVRD. In 9 patients with PR at enrollment, 1 achieved VGPR, but 8 remained in PR. Nevertheless, 2 out of 8 patients with PR after 6 courses of sVRD finally achieved VGPR or sCR after a total of 18 or 24 courses of sVRD, respectively. With a median follow-up time of 29.4 months, the median progression-free survival (PFS) and overall survival (OS) were not reached, while the PFS and OS rates at 2.5 years were 66.6% and 77.3%, respectively. Three patients died and their cause of death was disease progression in all cases. It is noteworthy that the three patients who discontinued sVRD treatment due to PD were the same three patients who died in spite of various post-study therapies. Univariate analysis demonstrated that disease progression as a reason for discontinuation of sVRD had a negative impact on OS. One dose modification of dexamethasone from 40 mg/day to 20 mg/day was required because of grade 2 hypertension after the 3rd course of sVRD. One patient had discontinued all study drugs because of grade 2 pneumonia after 6 courses of sVRD. There were no grade 3 or 4 hematologic or nonhematologic AEs. After enrollment, 2 new hematologic malignancies, i.e., ALL in one patient and myelodysplastic syndrome (MDS) in another patient, were diagnosed.
Conclusion
Our sVRD regimen as a consolidation/maintenance therapy was well-tolerable and highly effective in patients with MM who achieved at least PR after any induction therapy. We conclude that the dosage of bortezomib and lenalidomide in our sVRD regimen may be able to reduce AEs and have preserved efficacy simultaneously in the consolidation/maintenance setting.

Session topic: E-poster
Keyword(s): Consolidation, Maintenance, Multiple myeloma
{{ help_message }}
{{filter}}


