CARFILZOMIB, LENALIDOMIDE AND DEXAMETHASONE IN JAPANESE PATIENTS WITH RELAPSED OR REFRACTORY MULTIPLE MYELOMA
(Abstract release date: 05/19/16)
EHA Library. Ri M. 06/09/16; 134860; PB1960

Dr. Masaki Ri
Contributions
Contributions
Abstract
Abstract: PB1960
Type: Publication Only
Background
Carfilzomib (CFZ) is an epoxyketone proteasome inhibitor that binds selectively and irreversibly to the constitutive proteasome and immunoproteasome. The combination of CFZ with lenalidomide (Len) and dexamethasone (Dex) (CRd) has shown efficacy in a phase 3 study (ASPIRE) in relapsed multiple myeloma (Stewart et al, 2014).
Aims
This phase 1 study was designed to evaluate the safety, tolerability, efficacy and pharmacokinetics of CRd regimens in Japanese patients with relapsed or refractory multiple myeloma (RRMM). This was a company sponsored trial (Ono Pharmaceutical Co., Ltd.).
Methods
Adults with RRMM who had received at least 1 prior treatment were eligible. CFZ was administered as a 10-minute infusion on days 1, 2, 8, 9, 15, and 16 of 28-day treatment cycles (20 mg per square meter on days 1 and 2 of cycle 1 and 27 mg per square meter thereafter) during cycles 1 through 12 and on days 1, 2, 15, and 16 during cycles 13 through 18. Len (25 mg) was given on days 1 through 21 during cycles 1 through 18. Dex (40 mg) was administered on days 1, 8, 15, and 22 during cycles 1 through 18. The efficacy endpoint included the rate of overall response (ORR). Treatment responses and disease progression were assessed by investigators based on the central laboratory results with the IMWG Uniform Response Criteria. All patients provided written informed consent.
Results
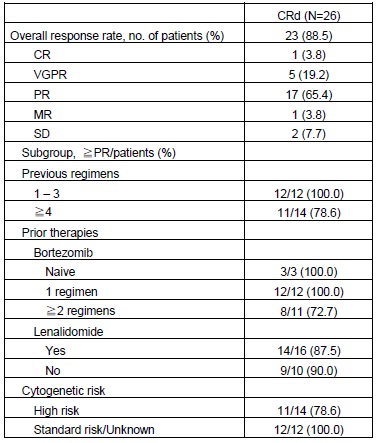
Twenty six Japanese RRMM patients were enrolled. The median number of previous regimens was 4 (range, 1-10). The median number of cycles dosed was 4 (range, 1-8 cycles). The proportion of patients with previous therapies of bortezomib and lenalidomide was 88.5% and 61.5%, respectively. A total of 53.8% of patients had high risk abnormal cytogenetics [t (4; 14), t (14; 16), del (17p) or hypodiploid]. In this study, CRd regimen used in ASPIRE study was well tolerated in Japanese population. The ORR was 88.5% (90%CI, 72.8 to 96.8). Subgroup analysis demonstrated that the ORR was not affected by previous therapies and abnormal cytogenetics. The median PFS and OS were not estimated because of the short follow-up period. The most common AEs included lymphocyte count decreased (53.8%), platelet count decreased (53.8%), hyperglycemia (38.5%), hypophosphatemia (38.5%), constipation (30.8%), white blood cell count decreased (30.8%), and rash (30.8%). The most common Grade 3 or higher AE were lymphocyte count decreased (42.3%), platelet count decreased (23.1%), hypophosphatemia (19.2%), anemia (11.5%), neutrophil count decreased (11.5%), white blood cell count decreased (11.5%) and hyperglycemia (11.5%). Peripheral Neuropathy was observed in 15.4% but no Grade ≥3 or peripheral neuropathy with pain was reported. None of the patients experienced interstitial lung disease. The plasma CFZ concentration showed a rapid decrease after intravenous administration with T1/2 of 0.580–0.740h. The exposure of CFZ increased in a dose-dependent manner with the AUCinf of 326-445 ng*h/mL, Cmax of 1540-2030 ng/mL.
Conclusion
This is the first study that CRd regimen was evaluated in heavily pre-treated MM patients (median 4 prior regimens). CRd regimen was well tolerated and showed a compelling efficacy in Japanese RRMM patients. A comparison of this study with CRd arm in ASPIRE phase 3 study showed that the results of the ORR were similar (88.5% and 87.1%), despite the more heavily pretreated patient population in this study (median 4 vs 2 prior regimens). The safety and efficacy of the CRd regimen in Japanese patients seems consistent with that reported in ASPIRE phase 3 study.
Session topic: E-poster
Keyword(s): Clinical trial, Imids, Multiple myeloma, Proteasome inhibitor
Type: Publication Only
Background
Carfilzomib (CFZ) is an epoxyketone proteasome inhibitor that binds selectively and irreversibly to the constitutive proteasome and immunoproteasome. The combination of CFZ with lenalidomide (Len) and dexamethasone (Dex) (CRd) has shown efficacy in a phase 3 study (ASPIRE) in relapsed multiple myeloma (Stewart et al, 2014).
Aims
This phase 1 study was designed to evaluate the safety, tolerability, efficacy and pharmacokinetics of CRd regimens in Japanese patients with relapsed or refractory multiple myeloma (RRMM). This was a company sponsored trial (Ono Pharmaceutical Co., Ltd.).
Methods
Adults with RRMM who had received at least 1 prior treatment were eligible. CFZ was administered as a 10-minute infusion on days 1, 2, 8, 9, 15, and 16 of 28-day treatment cycles (20 mg per square meter on days 1 and 2 of cycle 1 and 27 mg per square meter thereafter) during cycles 1 through 12 and on days 1, 2, 15, and 16 during cycles 13 through 18. Len (25 mg) was given on days 1 through 21 during cycles 1 through 18. Dex (40 mg) was administered on days 1, 8, 15, and 22 during cycles 1 through 18. The efficacy endpoint included the rate of overall response (ORR). Treatment responses and disease progression were assessed by investigators based on the central laboratory results with the IMWG Uniform Response Criteria. All patients provided written informed consent.
Results
Twenty six Japanese RRMM patients were enrolled. The median number of previous regimens was 4 (range, 1-10). The median number of cycles dosed was 4 (range, 1-8 cycles). The proportion of patients with previous therapies of bortezomib and lenalidomide was 88.5% and 61.5%, respectively. A total of 53.8% of patients had high risk abnormal cytogenetics [t (4; 14), t (14; 16), del (17p) or hypodiploid]. In this study, CRd regimen used in ASPIRE study was well tolerated in Japanese population. The ORR was 88.5% (90%CI, 72.8 to 96.8). Subgroup analysis demonstrated that the ORR was not affected by previous therapies and abnormal cytogenetics. The median PFS and OS were not estimated because of the short follow-up period. The most common AEs included lymphocyte count decreased (53.8%), platelet count decreased (53.8%), hyperglycemia (38.5%), hypophosphatemia (38.5%), constipation (30.8%), white blood cell count decreased (30.8%), and rash (30.8%). The most common Grade 3 or higher AE were lymphocyte count decreased (42.3%), platelet count decreased (23.1%), hypophosphatemia (19.2%), anemia (11.5%), neutrophil count decreased (11.5%), white blood cell count decreased (11.5%) and hyperglycemia (11.5%). Peripheral Neuropathy was observed in 15.4% but no Grade ≥3 or peripheral neuropathy with pain was reported. None of the patients experienced interstitial lung disease. The plasma CFZ concentration showed a rapid decrease after intravenous administration with T1/2 of 0.580–0.740h. The exposure of CFZ increased in a dose-dependent manner with the AUCinf of 326-445 ng*h/mL, Cmax of 1540-2030 ng/mL.
Conclusion
This is the first study that CRd regimen was evaluated in heavily pre-treated MM patients (median 4 prior regimens). CRd regimen was well tolerated and showed a compelling efficacy in Japanese RRMM patients. A comparison of this study with CRd arm in ASPIRE phase 3 study showed that the results of the ORR were similar (88.5% and 87.1%), despite the more heavily pretreated patient population in this study (median 4 vs 2 prior regimens). The safety and efficacy of the CRd regimen in Japanese patients seems consistent with that reported in ASPIRE phase 3 study.

Session topic: E-poster
Keyword(s): Clinical trial, Imids, Multiple myeloma, Proteasome inhibitor
Abstract: PB1960
Type: Publication Only
Background
Carfilzomib (CFZ) is an epoxyketone proteasome inhibitor that binds selectively and irreversibly to the constitutive proteasome and immunoproteasome. The combination of CFZ with lenalidomide (Len) and dexamethasone (Dex) (CRd) has shown efficacy in a phase 3 study (ASPIRE) in relapsed multiple myeloma (Stewart et al, 2014).
Aims
This phase 1 study was designed to evaluate the safety, tolerability, efficacy and pharmacokinetics of CRd regimens in Japanese patients with relapsed or refractory multiple myeloma (RRMM). This was a company sponsored trial (Ono Pharmaceutical Co., Ltd.).
Methods
Adults with RRMM who had received at least 1 prior treatment were eligible. CFZ was administered as a 10-minute infusion on days 1, 2, 8, 9, 15, and 16 of 28-day treatment cycles (20 mg per square meter on days 1 and 2 of cycle 1 and 27 mg per square meter thereafter) during cycles 1 through 12 and on days 1, 2, 15, and 16 during cycles 13 through 18. Len (25 mg) was given on days 1 through 21 during cycles 1 through 18. Dex (40 mg) was administered on days 1, 8, 15, and 22 during cycles 1 through 18. The efficacy endpoint included the rate of overall response (ORR). Treatment responses and disease progression were assessed by investigators based on the central laboratory results with the IMWG Uniform Response Criteria. All patients provided written informed consent.
Results
Twenty six Japanese RRMM patients were enrolled. The median number of previous regimens was 4 (range, 1-10). The median number of cycles dosed was 4 (range, 1-8 cycles). The proportion of patients with previous therapies of bortezomib and lenalidomide was 88.5% and 61.5%, respectively. A total of 53.8% of patients had high risk abnormal cytogenetics [t (4; 14), t (14; 16), del (17p) or hypodiploid]. In this study, CRd regimen used in ASPIRE study was well tolerated in Japanese population. The ORR was 88.5% (90%CI, 72.8 to 96.8). Subgroup analysis demonstrated that the ORR was not affected by previous therapies and abnormal cytogenetics. The median PFS and OS were not estimated because of the short follow-up period. The most common AEs included lymphocyte count decreased (53.8%), platelet count decreased (53.8%), hyperglycemia (38.5%), hypophosphatemia (38.5%), constipation (30.8%), white blood cell count decreased (30.8%), and rash (30.8%). The most common Grade 3 or higher AE were lymphocyte count decreased (42.3%), platelet count decreased (23.1%), hypophosphatemia (19.2%), anemia (11.5%), neutrophil count decreased (11.5%), white blood cell count decreased (11.5%) and hyperglycemia (11.5%). Peripheral Neuropathy was observed in 15.4% but no Grade ≥3 or peripheral neuropathy with pain was reported. None of the patients experienced interstitial lung disease. The plasma CFZ concentration showed a rapid decrease after intravenous administration with T1/2 of 0.580–0.740h. The exposure of CFZ increased in a dose-dependent manner with the AUCinf of 326-445 ng*h/mL, Cmax of 1540-2030 ng/mL.
Conclusion
This is the first study that CRd regimen was evaluated in heavily pre-treated MM patients (median 4 prior regimens). CRd regimen was well tolerated and showed a compelling efficacy in Japanese RRMM patients. A comparison of this study with CRd arm in ASPIRE phase 3 study showed that the results of the ORR were similar (88.5% and 87.1%), despite the more heavily pretreated patient population in this study (median 4 vs 2 prior regimens). The safety and efficacy of the CRd regimen in Japanese patients seems consistent with that reported in ASPIRE phase 3 study.

Session topic: E-poster
Keyword(s): Clinical trial, Imids, Multiple myeloma, Proteasome inhibitor
Type: Publication Only
Background
Carfilzomib (CFZ) is an epoxyketone proteasome inhibitor that binds selectively and irreversibly to the constitutive proteasome and immunoproteasome. The combination of CFZ with lenalidomide (Len) and dexamethasone (Dex) (CRd) has shown efficacy in a phase 3 study (ASPIRE) in relapsed multiple myeloma (Stewart et al, 2014).
Aims
This phase 1 study was designed to evaluate the safety, tolerability, efficacy and pharmacokinetics of CRd regimens in Japanese patients with relapsed or refractory multiple myeloma (RRMM). This was a company sponsored trial (Ono Pharmaceutical Co., Ltd.).
Methods
Adults with RRMM who had received at least 1 prior treatment were eligible. CFZ was administered as a 10-minute infusion on days 1, 2, 8, 9, 15, and 16 of 28-day treatment cycles (20 mg per square meter on days 1 and 2 of cycle 1 and 27 mg per square meter thereafter) during cycles 1 through 12 and on days 1, 2, 15, and 16 during cycles 13 through 18. Len (25 mg) was given on days 1 through 21 during cycles 1 through 18. Dex (40 mg) was administered on days 1, 8, 15, and 22 during cycles 1 through 18. The efficacy endpoint included the rate of overall response (ORR). Treatment responses and disease progression were assessed by investigators based on the central laboratory results with the IMWG Uniform Response Criteria. All patients provided written informed consent.
Results
Twenty six Japanese RRMM patients were enrolled. The median number of previous regimens was 4 (range, 1-10). The median number of cycles dosed was 4 (range, 1-8 cycles). The proportion of patients with previous therapies of bortezomib and lenalidomide was 88.5% and 61.5%, respectively. A total of 53.8% of patients had high risk abnormal cytogenetics [t (4; 14), t (14; 16), del (17p) or hypodiploid]. In this study, CRd regimen used in ASPIRE study was well tolerated in Japanese population. The ORR was 88.5% (90%CI, 72.8 to 96.8). Subgroup analysis demonstrated that the ORR was not affected by previous therapies and abnormal cytogenetics. The median PFS and OS were not estimated because of the short follow-up period. The most common AEs included lymphocyte count decreased (53.8%), platelet count decreased (53.8%), hyperglycemia (38.5%), hypophosphatemia (38.5%), constipation (30.8%), white blood cell count decreased (30.8%), and rash (30.8%). The most common Grade 3 or higher AE were lymphocyte count decreased (42.3%), platelet count decreased (23.1%), hypophosphatemia (19.2%), anemia (11.5%), neutrophil count decreased (11.5%), white blood cell count decreased (11.5%) and hyperglycemia (11.5%). Peripheral Neuropathy was observed in 15.4% but no Grade ≥3 or peripheral neuropathy with pain was reported. None of the patients experienced interstitial lung disease. The plasma CFZ concentration showed a rapid decrease after intravenous administration with T1/2 of 0.580–0.740h. The exposure of CFZ increased in a dose-dependent manner with the AUCinf of 326-445 ng*h/mL, Cmax of 1540-2030 ng/mL.
Conclusion
This is the first study that CRd regimen was evaluated in heavily pre-treated MM patients (median 4 prior regimens). CRd regimen was well tolerated and showed a compelling efficacy in Japanese RRMM patients. A comparison of this study with CRd arm in ASPIRE phase 3 study showed that the results of the ORR were similar (88.5% and 87.1%), despite the more heavily pretreated patient population in this study (median 4 vs 2 prior regimens). The safety and efficacy of the CRd regimen in Japanese patients seems consistent with that reported in ASPIRE phase 3 study.

Session topic: E-poster
Keyword(s): Clinical trial, Imids, Multiple myeloma, Proteasome inhibitor
{{ help_message }}
{{filter}}


