BIOSIMILAR ERYTHROPOIETIN ALFA FOR THE THERAPY OF ANEMIA IN ?LOWER RISK? MYELODYSPLASTIC SYNDROMES. INTERIM RESULTS FROM A PROSPECTIVE OBSERVATIONAL STUDY OF THE ?RETE EMATOLOGICA LOMBARDA? (EPOREL)
(Abstract release date: 05/19/16)
EHA Library. Maria Pelizzari A. 06/09/16; 134824; PB1924

Anna Maria Pelizzari
Contributions
Contributions
Abstract
Abstract: PB1924
Type: Publication Only
Background
Biosimilar EPO use for the treatment of anemia of MDS is based on therapeutic equivalence extrapolated from originator drug employed in other clinical settings; specific prospective evaluation of their activity in MDS are scarce. From November 2014 “lower risk” pts with newly-diagnosed MDS referred to the haematologic centres of REL and symptomatic anaemia participate to an observational multicenter prospective study on efficacy and safety of biosimilar EPO (EPOREL1 protocol).
Aims
1) Primary endpoint: to prospectively assess the response rate of “lower risk” MDS anaemic pts treated with biosimilar EPO alfa; 2) Secondary endpoint: to validate the prognostic power of Scandinavian Myelodysplasia Group score (SMG) (Hellstrom-Lindberg E et al, Br J Haematol 1997; 99:344) in this setting.
Methods
The study was in accordance with the ethical standards of the Committee of Human Experimentation of the coordinating center. MDS were diagnosed according to WHO 2008 criteria and classified according to IPSS and IPSS-R. EPO alfa was administered subcutaneously at the starting dose of 40.000 U/w. EPO was doubled in not responsive pts after 2 ms, reduced at mainteinance dose if hgb > 12 g/dl and stopped in case of refractoriness, relapse, AML evolution, death or major toxicity. Responses were defined by IWG criteria 2006.
Results
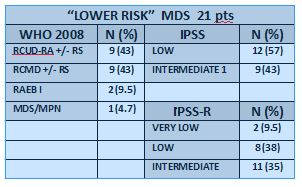
Data were collected from the charts of 26 consecutive pts treated with biosimilar EPO alfa; 21 were evaluable (five too early). Median age was 79 yrs (71-90); F/M 0,3. Their diagnostic and prognostic characteristics are described in Table 1. Twelve (57%) pts were transfusion dependent with median transfusion need of 2 units/ms (1-3). Median haemoglobin was 8,7 (7,5-9,8) and median endogeneous serum EPO, evaluable in 19 pts (90%), was 50 U/L (11-1410).Fifteen pts (71%) responded after a median time of 1 mo (1-10; D.S. 4,1, C.I.95%), 5/12 (42%) transfusion dependent pts reached transfusion independency. EPO dose was incremented to 80.000U/w in 13 pts (62%) after a median of 2 ms (1-8). After a median f.up of 6 ms (2-15), all responsive pts maintained the response and are still on treatment.Ten of 11 (91%) pts with SMG score good and 1/6 (17%) pts with SMG score intermediate achieved an erythroid response (p=0.005). No adverse events were reported up to now.AML evolution was documented in three non responsive cases after a median follow-up of 3 ms (2-6): two pts were off therapy; one case IPSS int1, IPSS-R int, normal karyotype, evolved during treatment.Two pts died: one off-therapy after AML transformation for septic shock, one responsive with previous cardiopathy for congestive hearth failure.
Conclusion
To our knowledge this is the first prospective study on MDS pts treatment with biosimilar EPO alfa (HX575). The response rate and safety profile of biosimilar EPO alfa is comparable with published data on originator EPO, even in this older MDS cohort. The study confirms the prognostic power of SMG score in this setting. Further evaluation after prolonged follow-up on larger series of pts is warranted.
Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Myelodysplasia
Type: Publication Only
Background
Biosimilar EPO use for the treatment of anemia of MDS is based on therapeutic equivalence extrapolated from originator drug employed in other clinical settings; specific prospective evaluation of their activity in MDS are scarce. From November 2014 “lower risk” pts with newly-diagnosed MDS referred to the haematologic centres of REL and symptomatic anaemia participate to an observational multicenter prospective study on efficacy and safety of biosimilar EPO (EPOREL1 protocol).
Aims
1) Primary endpoint: to prospectively assess the response rate of “lower risk” MDS anaemic pts treated with biosimilar EPO alfa; 2) Secondary endpoint: to validate the prognostic power of Scandinavian Myelodysplasia Group score (SMG) (Hellstrom-Lindberg E et al, Br J Haematol 1997; 99:344) in this setting.
Methods
The study was in accordance with the ethical standards of the Committee of Human Experimentation of the coordinating center. MDS were diagnosed according to WHO 2008 criteria and classified according to IPSS and IPSS-R. EPO alfa was administered subcutaneously at the starting dose of 40.000 U/w. EPO was doubled in not responsive pts after 2 ms, reduced at mainteinance dose if hgb > 12 g/dl and stopped in case of refractoriness, relapse, AML evolution, death or major toxicity. Responses were defined by IWG criteria 2006.
Results
Data were collected from the charts of 26 consecutive pts treated with biosimilar EPO alfa; 21 were evaluable (five too early). Median age was 79 yrs (71-90); F/M 0,3. Their diagnostic and prognostic characteristics are described in Table 1. Twelve (57%) pts were transfusion dependent with median transfusion need of 2 units/ms (1-3). Median haemoglobin was 8,7 (7,5-9,8) and median endogeneous serum EPO, evaluable in 19 pts (90%), was 50 U/L (11-1410).Fifteen pts (71%) responded after a median time of 1 mo (1-10; D.S. 4,1, C.I.95%), 5/12 (42%) transfusion dependent pts reached transfusion independency. EPO dose was incremented to 80.000U/w in 13 pts (62%) after a median of 2 ms (1-8). After a median f.up of 6 ms (2-15), all responsive pts maintained the response and are still on treatment.Ten of 11 (91%) pts with SMG score good and 1/6 (17%) pts with SMG score intermediate achieved an erythroid response (p=0.005). No adverse events were reported up to now.AML evolution was documented in three non responsive cases after a median follow-up of 3 ms (2-6): two pts were off therapy; one case IPSS int1, IPSS-R int, normal karyotype, evolved during treatment.Two pts died: one off-therapy after AML transformation for septic shock, one responsive with previous cardiopathy for congestive hearth failure.
Conclusion
To our knowledge this is the first prospective study on MDS pts treatment with biosimilar EPO alfa (HX575). The response rate and safety profile of biosimilar EPO alfa is comparable with published data on originator EPO, even in this older MDS cohort. The study confirms the prognostic power of SMG score in this setting. Further evaluation after prolonged follow-up on larger series of pts is warranted.

Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Myelodysplasia
Abstract: PB1924
Type: Publication Only
Background
Biosimilar EPO use for the treatment of anemia of MDS is based on therapeutic equivalence extrapolated from originator drug employed in other clinical settings; specific prospective evaluation of their activity in MDS are scarce. From November 2014 “lower risk” pts with newly-diagnosed MDS referred to the haematologic centres of REL and symptomatic anaemia participate to an observational multicenter prospective study on efficacy and safety of biosimilar EPO (EPOREL1 protocol).
Aims
1) Primary endpoint: to prospectively assess the response rate of “lower risk” MDS anaemic pts treated with biosimilar EPO alfa; 2) Secondary endpoint: to validate the prognostic power of Scandinavian Myelodysplasia Group score (SMG) (Hellstrom-Lindberg E et al, Br J Haematol 1997; 99:344) in this setting.
Methods
The study was in accordance with the ethical standards of the Committee of Human Experimentation of the coordinating center. MDS were diagnosed according to WHO 2008 criteria and classified according to IPSS and IPSS-R. EPO alfa was administered subcutaneously at the starting dose of 40.000 U/w. EPO was doubled in not responsive pts after 2 ms, reduced at mainteinance dose if hgb > 12 g/dl and stopped in case of refractoriness, relapse, AML evolution, death or major toxicity. Responses were defined by IWG criteria 2006.
Results
Data were collected from the charts of 26 consecutive pts treated with biosimilar EPO alfa; 21 were evaluable (five too early). Median age was 79 yrs (71-90); F/M 0,3. Their diagnostic and prognostic characteristics are described in Table 1. Twelve (57%) pts were transfusion dependent with median transfusion need of 2 units/ms (1-3). Median haemoglobin was 8,7 (7,5-9,8) and median endogeneous serum EPO, evaluable in 19 pts (90%), was 50 U/L (11-1410).Fifteen pts (71%) responded after a median time of 1 mo (1-10; D.S. 4,1, C.I.95%), 5/12 (42%) transfusion dependent pts reached transfusion independency. EPO dose was incremented to 80.000U/w in 13 pts (62%) after a median of 2 ms (1-8). After a median f.up of 6 ms (2-15), all responsive pts maintained the response and are still on treatment.Ten of 11 (91%) pts with SMG score good and 1/6 (17%) pts with SMG score intermediate achieved an erythroid response (p=0.005). No adverse events were reported up to now.AML evolution was documented in three non responsive cases after a median follow-up of 3 ms (2-6): two pts were off therapy; one case IPSS int1, IPSS-R int, normal karyotype, evolved during treatment.Two pts died: one off-therapy after AML transformation for septic shock, one responsive with previous cardiopathy for congestive hearth failure.
Conclusion
To our knowledge this is the first prospective study on MDS pts treatment with biosimilar EPO alfa (HX575). The response rate and safety profile of biosimilar EPO alfa is comparable with published data on originator EPO, even in this older MDS cohort. The study confirms the prognostic power of SMG score in this setting. Further evaluation after prolonged follow-up on larger series of pts is warranted.

Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Myelodysplasia
Type: Publication Only
Background
Biosimilar EPO use for the treatment of anemia of MDS is based on therapeutic equivalence extrapolated from originator drug employed in other clinical settings; specific prospective evaluation of their activity in MDS are scarce. From November 2014 “lower risk” pts with newly-diagnosed MDS referred to the haematologic centres of REL and symptomatic anaemia participate to an observational multicenter prospective study on efficacy and safety of biosimilar EPO (EPOREL1 protocol).
Aims
1) Primary endpoint: to prospectively assess the response rate of “lower risk” MDS anaemic pts treated with biosimilar EPO alfa; 2) Secondary endpoint: to validate the prognostic power of Scandinavian Myelodysplasia Group score (SMG) (Hellstrom-Lindberg E et al, Br J Haematol 1997; 99:344) in this setting.
Methods
The study was in accordance with the ethical standards of the Committee of Human Experimentation of the coordinating center. MDS were diagnosed according to WHO 2008 criteria and classified according to IPSS and IPSS-R. EPO alfa was administered subcutaneously at the starting dose of 40.000 U/w. EPO was doubled in not responsive pts after 2 ms, reduced at mainteinance dose if hgb > 12 g/dl and stopped in case of refractoriness, relapse, AML evolution, death or major toxicity. Responses were defined by IWG criteria 2006.
Results
Data were collected from the charts of 26 consecutive pts treated with biosimilar EPO alfa; 21 were evaluable (five too early). Median age was 79 yrs (71-90); F/M 0,3. Their diagnostic and prognostic characteristics are described in Table 1. Twelve (57%) pts were transfusion dependent with median transfusion need of 2 units/ms (1-3). Median haemoglobin was 8,7 (7,5-9,8) and median endogeneous serum EPO, evaluable in 19 pts (90%), was 50 U/L (11-1410).Fifteen pts (71%) responded after a median time of 1 mo (1-10; D.S. 4,1, C.I.95%), 5/12 (42%) transfusion dependent pts reached transfusion independency. EPO dose was incremented to 80.000U/w in 13 pts (62%) after a median of 2 ms (1-8). After a median f.up of 6 ms (2-15), all responsive pts maintained the response and are still on treatment.Ten of 11 (91%) pts with SMG score good and 1/6 (17%) pts with SMG score intermediate achieved an erythroid response (p=0.005). No adverse events were reported up to now.AML evolution was documented in three non responsive cases after a median follow-up of 3 ms (2-6): two pts were off therapy; one case IPSS int1, IPSS-R int, normal karyotype, evolved during treatment.Two pts died: one off-therapy after AML transformation for septic shock, one responsive with previous cardiopathy for congestive hearth failure.
Conclusion
To our knowledge this is the first prospective study on MDS pts treatment with biosimilar EPO alfa (HX575). The response rate and safety profile of biosimilar EPO alfa is comparable with published data on originator EPO, even in this older MDS cohort. The study confirms the prognostic power of SMG score in this setting. Further evaluation after prolonged follow-up on larger series of pts is warranted.

Session topic: E-poster
Keyword(s): Anemia, Erythropoietin, Myelodysplasia
{{ help_message }}
{{filter}}


