PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH) SCREENING IN PATIENTS WITH MYELODYSPLASTIC SYNDROME (MDS) IN CLINICAL PRACTICE: FREQUENCY AND INDICATIONS
(Abstract release date: 05/19/16)
EHA Library. Wong S. 06/09/16; 134819; PB1919

Prof. Dr. Shannon Wong
Contributions
Contributions
Abstract
Abstract: PB1919
Type: Publication Only
Background
MDS is a group of bone marrow disorders characterized by ineffective hematopoiesis leading to peripheral blood cytopenias. Most MDS patients (pts) develop significant anemia and red blood cell (RBC) transfusion dependence (TD). In PNH, mutations in the phosphatidyl inositol glycan (PIGA) gene lead to lack of the glycosylphosphatidyl inositol (GPI) anchor on the cell surface allowing complement-mediated lysis to occur. The PNH phenotype includes direct antiglobulin test (DAT) negative hemolysis and cytopenias including TD anemia, hemoglobinuria (resulting in iron deficiency in some pts), and thrombosis. PNH clones are detected in up to 50% of MDS pts, might confound the reason for RBC TD and PNH+ MDS pts may have better response to immunosuppressive therapy (IST). Eculizumab is the first specific treatment for PNH approved in Canada (2009). It reduces hemolysis and RBC transfusion requirements, prevents thrombosis, improves renal function, quality of life and overall survival (OS).We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Aims
We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Methods
All pts with MDS seen at St. Paul's Hospital were reviewed. Only pts with a bone marrow biopsy confirmed MDS diagnosis (dx) since 2009 were included. Data extracted were baseline clinical and laboratory features, clinical course, treatment and outcome. Indicators of hemolysis including increased levels of lactate dehydrogenase (LDH), bilirubin (BILI) andreticulocyte count (RETICS), decreased haptogobin (HAPTO), and results of DAT testing were recorded. High resolution PNH testing was done by flow cytometry for expression of FLAER, CD24, CD14, and CD59 on neutrophils, monocytes and RBC.
Results
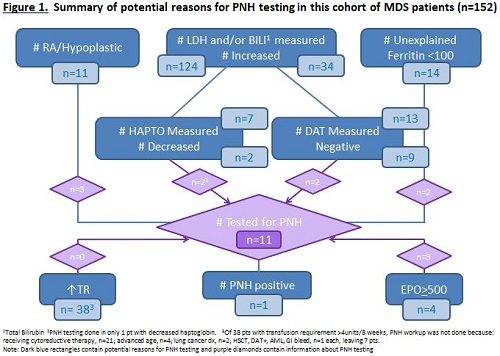
Of 395 MDS pts, 152 were diagnosed in 2009 or later. Median age at MDS dx was 73.5 (range 38-91) years and 66% were male. MDS dx was: RA, n=7; RARS, n=17; RCMD±RS, n=40; RAEB, n=23; hypoplastic, n=4; other, n=61. IPSS scores were: low, n=53; int-1, n=59; int-2, n=32; and high, n=8. MDS treatment was supportive care only in 53 pts. The erythropoietin (EPO) level was > 500mIU/mL in 4 pts. LDH, BILI and RETICS were measured (and elevated) in 96 (23), 109 (10) and 142 (14) pts, respectively and HAPTO was decreased in 2 of 7 measured. DAT was negative in 9 of 13 pts and serum ferritin level <100ng/mL in 14 of 116. No pts had hemoglobinuria or thrombosis. The hemoglobin was <100G/L in 86 at MDS dx. 79 (52%) pts were RBC TD, with a median transfusion requirement of 4 (1-8) units/8 weeks. PNH testing was positive in 1 of 11 pts tested. Reasons for PNH testing were: anemia, n=3 (with abdominal symptoms and pre-MDS dx, n=1 each); new MDS dx, n=2;hypoplastic MDS, n=2; decreased HAPTO; increased RBC transfusion requirements; and iron deficiency, n=1 each; see Figure and Table. Of patients with an RBC transfusion requirement >4 units/8 weeks none underwent PNH testing. At a median follow up of 21.1 (0.7-69.9) months for all patients, 113 were alive and the median OS was not reached.
Conclusion
PNH was tested for infrequently in MDS patients in clinical practice. Only 11 (7%) of MDS pts since 2009 had PNH testing done despite potential indicators of hemolysis in 27%. Clinical rather than laboratory indicators prompted PNH testing in 6 of 11 pts. Although the clinical significance of PNH clones in MDS is not yet fully defined, complement mediated hemolysis could exacerbate anemia. As there is now an effective treatment available, and PNH+ MDS pts may respond to IST, screening for PNH in MDS should be considered.
Session topic: E-poster
Keyword(s): Myelodysplasia, Paroxysmal nocturnal hemoglobinuria (PNH), Screening
Type: Publication Only
Background
MDS is a group of bone marrow disorders characterized by ineffective hematopoiesis leading to peripheral blood cytopenias. Most MDS patients (pts) develop significant anemia and red blood cell (RBC) transfusion dependence (TD). In PNH, mutations in the phosphatidyl inositol glycan (PIGA) gene lead to lack of the glycosylphosphatidyl inositol (GPI) anchor on the cell surface allowing complement-mediated lysis to occur. The PNH phenotype includes direct antiglobulin test (DAT) negative hemolysis and cytopenias including TD anemia, hemoglobinuria (resulting in iron deficiency in some pts), and thrombosis. PNH clones are detected in up to 50% of MDS pts, might confound the reason for RBC TD and PNH+ MDS pts may have better response to immunosuppressive therapy (IST). Eculizumab is the first specific treatment for PNH approved in Canada (2009). It reduces hemolysis and RBC transfusion requirements, prevents thrombosis, improves renal function, quality of life and overall survival (OS).We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Aims
We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Methods
All pts with MDS seen at St. Paul's Hospital were reviewed. Only pts with a bone marrow biopsy confirmed MDS diagnosis (dx) since 2009 were included. Data extracted were baseline clinical and laboratory features, clinical course, treatment and outcome. Indicators of hemolysis including increased levels of lactate dehydrogenase (LDH), bilirubin (BILI) andreticulocyte count (RETICS), decreased haptogobin (HAPTO), and results of DAT testing were recorded. High resolution PNH testing was done by flow cytometry for expression of FLAER, CD24, CD14, and CD59 on neutrophils, monocytes and RBC.
Results
Of 395 MDS pts, 152 were diagnosed in 2009 or later. Median age at MDS dx was 73.5 (range 38-91) years and 66% were male. MDS dx was: RA, n=7; RARS, n=17; RCMD±RS, n=40; RAEB, n=23; hypoplastic, n=4; other, n=61. IPSS scores were: low, n=53; int-1, n=59; int-2, n=32; and high, n=8. MDS treatment was supportive care only in 53 pts. The erythropoietin (EPO) level was > 500mIU/mL in 4 pts. LDH, BILI and RETICS were measured (and elevated) in 96 (23), 109 (10) and 142 (14) pts, respectively and HAPTO was decreased in 2 of 7 measured. DAT was negative in 9 of 13 pts and serum ferritin level <100ng/mL in 14 of 116. No pts had hemoglobinuria or thrombosis. The hemoglobin was <100G/L in 86 at MDS dx. 79 (52%) pts were RBC TD, with a median transfusion requirement of 4 (1-8) units/8 weeks. PNH testing was positive in 1 of 11 pts tested. Reasons for PNH testing were: anemia, n=3 (with abdominal symptoms and pre-MDS dx, n=1 each); new MDS dx, n=2;hypoplastic MDS, n=2; decreased HAPTO; increased RBC transfusion requirements; and iron deficiency, n=1 each; see Figure and Table. Of patients with an RBC transfusion requirement >4 units/8 weeks none underwent PNH testing. At a median follow up of 21.1 (0.7-69.9) months for all patients, 113 were alive and the median OS was not reached.
Conclusion
PNH was tested for infrequently in MDS patients in clinical practice. Only 11 (7%) of MDS pts since 2009 had PNH testing done despite potential indicators of hemolysis in 27%. Clinical rather than laboratory indicators prompted PNH testing in 6 of 11 pts. Although the clinical significance of PNH clones in MDS is not yet fully defined, complement mediated hemolysis could exacerbate anemia. As there is now an effective treatment available, and PNH+ MDS pts may respond to IST, screening for PNH in MDS should be considered.

Session topic: E-poster
Keyword(s): Myelodysplasia, Paroxysmal nocturnal hemoglobinuria (PNH), Screening
Abstract: PB1919
Type: Publication Only
Background
MDS is a group of bone marrow disorders characterized by ineffective hematopoiesis leading to peripheral blood cytopenias. Most MDS patients (pts) develop significant anemia and red blood cell (RBC) transfusion dependence (TD). In PNH, mutations in the phosphatidyl inositol glycan (PIGA) gene lead to lack of the glycosylphosphatidyl inositol (GPI) anchor on the cell surface allowing complement-mediated lysis to occur. The PNH phenotype includes direct antiglobulin test (DAT) negative hemolysis and cytopenias including TD anemia, hemoglobinuria (resulting in iron deficiency in some pts), and thrombosis. PNH clones are detected in up to 50% of MDS pts, might confound the reason for RBC TD and PNH+ MDS pts may have better response to immunosuppressive therapy (IST). Eculizumab is the first specific treatment for PNH approved in Canada (2009). It reduces hemolysis and RBC transfusion requirements, prevents thrombosis, improves renal function, quality of life and overall survival (OS).We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Aims
We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Methods
All pts with MDS seen at St. Paul's Hospital were reviewed. Only pts with a bone marrow biopsy confirmed MDS diagnosis (dx) since 2009 were included. Data extracted were baseline clinical and laboratory features, clinical course, treatment and outcome. Indicators of hemolysis including increased levels of lactate dehydrogenase (LDH), bilirubin (BILI) andreticulocyte count (RETICS), decreased haptogobin (HAPTO), and results of DAT testing were recorded. High resolution PNH testing was done by flow cytometry for expression of FLAER, CD24, CD14, and CD59 on neutrophils, monocytes and RBC.
Results
Of 395 MDS pts, 152 were diagnosed in 2009 or later. Median age at MDS dx was 73.5 (range 38-91) years and 66% were male. MDS dx was: RA, n=7; RARS, n=17; RCMD±RS, n=40; RAEB, n=23; hypoplastic, n=4; other, n=61. IPSS scores were: low, n=53; int-1, n=59; int-2, n=32; and high, n=8. MDS treatment was supportive care only in 53 pts. The erythropoietin (EPO) level was > 500mIU/mL in 4 pts. LDH, BILI and RETICS were measured (and elevated) in 96 (23), 109 (10) and 142 (14) pts, respectively and HAPTO was decreased in 2 of 7 measured. DAT was negative in 9 of 13 pts and serum ferritin level <100ng/mL in 14 of 116. No pts had hemoglobinuria or thrombosis. The hemoglobin was <100G/L in 86 at MDS dx. 79 (52%) pts were RBC TD, with a median transfusion requirement of 4 (1-8) units/8 weeks. PNH testing was positive in 1 of 11 pts tested. Reasons for PNH testing were: anemia, n=3 (with abdominal symptoms and pre-MDS dx, n=1 each); new MDS dx, n=2;hypoplastic MDS, n=2; decreased HAPTO; increased RBC transfusion requirements; and iron deficiency, n=1 each; see Figure and Table. Of patients with an RBC transfusion requirement >4 units/8 weeks none underwent PNH testing. At a median follow up of 21.1 (0.7-69.9) months for all patients, 113 were alive and the median OS was not reached.
Conclusion
PNH was tested for infrequently in MDS patients in clinical practice. Only 11 (7%) of MDS pts since 2009 had PNH testing done despite potential indicators of hemolysis in 27%. Clinical rather than laboratory indicators prompted PNH testing in 6 of 11 pts. Although the clinical significance of PNH clones in MDS is not yet fully defined, complement mediated hemolysis could exacerbate anemia. As there is now an effective treatment available, and PNH+ MDS pts may respond to IST, screening for PNH in MDS should be considered.

Session topic: E-poster
Keyword(s): Myelodysplasia, Paroxysmal nocturnal hemoglobinuria (PNH), Screening
Type: Publication Only
Background
MDS is a group of bone marrow disorders characterized by ineffective hematopoiesis leading to peripheral blood cytopenias. Most MDS patients (pts) develop significant anemia and red blood cell (RBC) transfusion dependence (TD). In PNH, mutations in the phosphatidyl inositol glycan (PIGA) gene lead to lack of the glycosylphosphatidyl inositol (GPI) anchor on the cell surface allowing complement-mediated lysis to occur. The PNH phenotype includes direct antiglobulin test (DAT) negative hemolysis and cytopenias including TD anemia, hemoglobinuria (resulting in iron deficiency in some pts), and thrombosis. PNH clones are detected in up to 50% of MDS pts, might confound the reason for RBC TD and PNH+ MDS pts may have better response to immunosuppressive therapy (IST). Eculizumab is the first specific treatment for PNH approved in Canada (2009). It reduces hemolysis and RBC transfusion requirements, prevents thrombosis, improves renal function, quality of life and overall survival (OS).We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Aims
We wanted to determine whether PNH as a contributor to anemia is considered in MDS pts.
Methods
All pts with MDS seen at St. Paul's Hospital were reviewed. Only pts with a bone marrow biopsy confirmed MDS diagnosis (dx) since 2009 were included. Data extracted were baseline clinical and laboratory features, clinical course, treatment and outcome. Indicators of hemolysis including increased levels of lactate dehydrogenase (LDH), bilirubin (BILI) andreticulocyte count (RETICS), decreased haptogobin (HAPTO), and results of DAT testing were recorded. High resolution PNH testing was done by flow cytometry for expression of FLAER, CD24, CD14, and CD59 on neutrophils, monocytes and RBC.
Results
Of 395 MDS pts, 152 were diagnosed in 2009 or later. Median age at MDS dx was 73.5 (range 38-91) years and 66% were male. MDS dx was: RA, n=7; RARS, n=17; RCMD±RS, n=40; RAEB, n=23; hypoplastic, n=4; other, n=61. IPSS scores were: low, n=53; int-1, n=59; int-2, n=32; and high, n=8. MDS treatment was supportive care only in 53 pts. The erythropoietin (EPO) level was > 500mIU/mL in 4 pts. LDH, BILI and RETICS were measured (and elevated) in 96 (23), 109 (10) and 142 (14) pts, respectively and HAPTO was decreased in 2 of 7 measured. DAT was negative in 9 of 13 pts and serum ferritin level <100ng/mL in 14 of 116. No pts had hemoglobinuria or thrombosis. The hemoglobin was <100G/L in 86 at MDS dx. 79 (52%) pts were RBC TD, with a median transfusion requirement of 4 (1-8) units/8 weeks. PNH testing was positive in 1 of 11 pts tested. Reasons for PNH testing were: anemia, n=3 (with abdominal symptoms and pre-MDS dx, n=1 each); new MDS dx, n=2;hypoplastic MDS, n=2; decreased HAPTO; increased RBC transfusion requirements; and iron deficiency, n=1 each; see Figure and Table. Of patients with an RBC transfusion requirement >4 units/8 weeks none underwent PNH testing. At a median follow up of 21.1 (0.7-69.9) months for all patients, 113 were alive and the median OS was not reached.
Conclusion
PNH was tested for infrequently in MDS patients in clinical practice. Only 11 (7%) of MDS pts since 2009 had PNH testing done despite potential indicators of hemolysis in 27%. Clinical rather than laboratory indicators prompted PNH testing in 6 of 11 pts. Although the clinical significance of PNH clones in MDS is not yet fully defined, complement mediated hemolysis could exacerbate anemia. As there is now an effective treatment available, and PNH+ MDS pts may respond to IST, screening for PNH in MDS should be considered.

Session topic: E-poster
Keyword(s): Myelodysplasia, Paroxysmal nocturnal hemoglobinuria (PNH), Screening
{{ help_message }}
{{filter}}


