THE RESULTS OF TYROSINE-KINASE INHIBITORS TREATMENT IN CHRONIC MYELOID LEUKEMIA PATIENTS ACCORDING TO 2013 EUROPEAN LEUKEMIANET RESPONSE CRITERIA: RUSSIAN SINGLE-CENTER STUDY
(Abstract release date: 05/19/16)
EHA Library. Shukhov O. 06/09/16; 134742; PB1842

Dr. Oleg Shukhov
Contributions
Contributions
Abstract
Abstract: PB1842
Type: Publication Only
Background
The 2013 European LeukemiaNet (ELN) response criteria define when to change therapy in the case of treatment failure, when the treatment with tyrosine-kinase inhibitors (TKIs) of CML patients should be continued (optimal response) and when a careful monitoring is required (warning). To date, no data presented that show the results of the treatment approach according to ELN2013 response criteria.
Aims
The aim of the study was to evaluate the results of TKIs treatment in CML patients according to ELN2013 recommendations.
Methods
The prospective study included 71 adult patients with newly diagnosed CML in chronic phase. Baseline demographics characteristics: median age: 44 years (interquartile range (IQR) 31-57 years); male sex: 51% (n=36); Sokal score: high 23%, intermediate 27%, low 50%. Baseline treatment: imatinib (IM) 400 mg (n=63), nilotinib (NIL) 600 mg (n=7), dasatinib (DAS) 100 mg (n=1). The switching to another TKI or increasing doses was performed at 3,6,12 months or late in case of failure according to ELN2013 recommendations. Therapy was also changed in cases with no early molecular response (EMR) (BCR-ABL ≤10% at 3 months). Overall survival (OS), cumulative incidence of complete cytogenetic response (CCyR), major molecular response (MMR) and deep molecular response (MR4 or deeper) were evaluated (Intention-to-treat analysis).
Results
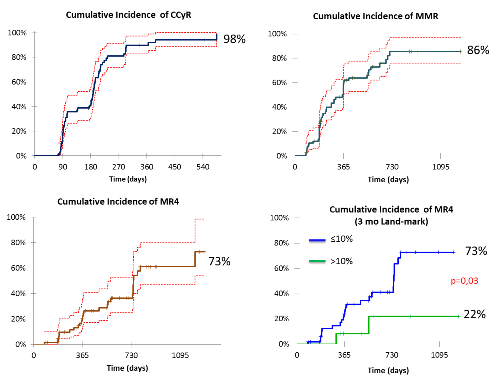
The failure was recorded in 14 (20%) cases (at 3 months n=6, at 6 months n=3, at 12 months n=2, after 12 months n=2, 1 patient died before 3 month assessment due to progression). Imatinib was a baseline treatment in all failures. Therapeutic options for failures: increasing dose of IM (600 mg) n=2, switching to DAS n=5, switching to NIL n=4, 2 patients are currently being examined before switching. In 6 cases switching or IM dose escalation was performed because of no EMR (out of failure) (NIL n=3, DAS n=1, IM600 mg n=2), other reasons (toxicity, late warning): n=4 (NIL n=2, DAS n=2). Median time to switching after failure detection was 67 days (IQR 36-103 days). Median follow-up: 19 months (IQR 12-31 months). Still alive: n=69; on IM treatment: n=44 (64%), on 2nd TKI: n=25 (36%). The 3-yars OS was 96%. Cumulative incidence of CCyR, MMR and MR4 was 98%, 86% and 73% respectively. Independent of switching, cumulative incidence of MR4 was higher in group with EMR than no EMR: 73% and 22%, respectively (p=0,03) (3 months landmark).
Conclusion
The principle of early therapy modification in patients with no optimal response according to ELN2013 response criteria allows to reach CCyR (the main surrogate endpoint for survival) in almost all cases. Early molecular response is a predictor of achieving deeper molecular response not depending on early switching approach.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Clinical outcome, Molecular response, Therapy
Type: Publication Only
Background
The 2013 European LeukemiaNet (ELN) response criteria define when to change therapy in the case of treatment failure, when the treatment with tyrosine-kinase inhibitors (TKIs) of CML patients should be continued (optimal response) and when a careful monitoring is required (warning). To date, no data presented that show the results of the treatment approach according to ELN2013 response criteria.
Aims
The aim of the study was to evaluate the results of TKIs treatment in CML patients according to ELN2013 recommendations.
Methods
The prospective study included 71 adult patients with newly diagnosed CML in chronic phase. Baseline demographics characteristics: median age: 44 years (interquartile range (IQR) 31-57 years); male sex: 51% (n=36); Sokal score: high 23%, intermediate 27%, low 50%. Baseline treatment: imatinib (IM) 400 mg (n=63), nilotinib (NIL) 600 mg (n=7), dasatinib (DAS) 100 mg (n=1). The switching to another TKI or increasing doses was performed at 3,6,12 months or late in case of failure according to ELN2013 recommendations. Therapy was also changed in cases with no early molecular response (EMR) (BCR-ABL ≤10% at 3 months). Overall survival (OS), cumulative incidence of complete cytogenetic response (CCyR), major molecular response (MMR) and deep molecular response (MR4 or deeper) were evaluated (Intention-to-treat analysis).
Results
The failure was recorded in 14 (20%) cases (at 3 months n=6, at 6 months n=3, at 12 months n=2, after 12 months n=2, 1 patient died before 3 month assessment due to progression). Imatinib was a baseline treatment in all failures. Therapeutic options for failures: increasing dose of IM (600 mg) n=2, switching to DAS n=5, switching to NIL n=4, 2 patients are currently being examined before switching. In 6 cases switching or IM dose escalation was performed because of no EMR (out of failure) (NIL n=3, DAS n=1, IM600 mg n=2), other reasons (toxicity, late warning): n=4 (NIL n=2, DAS n=2). Median time to switching after failure detection was 67 days (IQR 36-103 days). Median follow-up: 19 months (IQR 12-31 months). Still alive: n=69; on IM treatment: n=44 (64%), on 2nd TKI: n=25 (36%). The 3-yars OS was 96%. Cumulative incidence of CCyR, MMR and MR4 was 98%, 86% and 73% respectively. Independent of switching, cumulative incidence of MR4 was higher in group with EMR than no EMR: 73% and 22%, respectively (p=0,03) (3 months landmark).
Conclusion
The principle of early therapy modification in patients with no optimal response according to ELN2013 response criteria allows to reach CCyR (the main surrogate endpoint for survival) in almost all cases. Early molecular response is a predictor of achieving deeper molecular response not depending on early switching approach.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Clinical outcome, Molecular response, Therapy
Abstract: PB1842
Type: Publication Only
Background
The 2013 European LeukemiaNet (ELN) response criteria define when to change therapy in the case of treatment failure, when the treatment with tyrosine-kinase inhibitors (TKIs) of CML patients should be continued (optimal response) and when a careful monitoring is required (warning). To date, no data presented that show the results of the treatment approach according to ELN2013 response criteria.
Aims
The aim of the study was to evaluate the results of TKIs treatment in CML patients according to ELN2013 recommendations.
Methods
The prospective study included 71 adult patients with newly diagnosed CML in chronic phase. Baseline demographics characteristics: median age: 44 years (interquartile range (IQR) 31-57 years); male sex: 51% (n=36); Sokal score: high 23%, intermediate 27%, low 50%. Baseline treatment: imatinib (IM) 400 mg (n=63), nilotinib (NIL) 600 mg (n=7), dasatinib (DAS) 100 mg (n=1). The switching to another TKI or increasing doses was performed at 3,6,12 months or late in case of failure according to ELN2013 recommendations. Therapy was also changed in cases with no early molecular response (EMR) (BCR-ABL ≤10% at 3 months). Overall survival (OS), cumulative incidence of complete cytogenetic response (CCyR), major molecular response (MMR) and deep molecular response (MR4 or deeper) were evaluated (Intention-to-treat analysis).
Results
The failure was recorded in 14 (20%) cases (at 3 months n=6, at 6 months n=3, at 12 months n=2, after 12 months n=2, 1 patient died before 3 month assessment due to progression). Imatinib was a baseline treatment in all failures. Therapeutic options for failures: increasing dose of IM (600 mg) n=2, switching to DAS n=5, switching to NIL n=4, 2 patients are currently being examined before switching. In 6 cases switching or IM dose escalation was performed because of no EMR (out of failure) (NIL n=3, DAS n=1, IM600 mg n=2), other reasons (toxicity, late warning): n=4 (NIL n=2, DAS n=2). Median time to switching after failure detection was 67 days (IQR 36-103 days). Median follow-up: 19 months (IQR 12-31 months). Still alive: n=69; on IM treatment: n=44 (64%), on 2nd TKI: n=25 (36%). The 3-yars OS was 96%. Cumulative incidence of CCyR, MMR and MR4 was 98%, 86% and 73% respectively. Independent of switching, cumulative incidence of MR4 was higher in group with EMR than no EMR: 73% and 22%, respectively (p=0,03) (3 months landmark).
Conclusion
The principle of early therapy modification in patients with no optimal response according to ELN2013 response criteria allows to reach CCyR (the main surrogate endpoint for survival) in almost all cases. Early molecular response is a predictor of achieving deeper molecular response not depending on early switching approach.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Clinical outcome, Molecular response, Therapy
Type: Publication Only
Background
The 2013 European LeukemiaNet (ELN) response criteria define when to change therapy in the case of treatment failure, when the treatment with tyrosine-kinase inhibitors (TKIs) of CML patients should be continued (optimal response) and when a careful monitoring is required (warning). To date, no data presented that show the results of the treatment approach according to ELN2013 response criteria.
Aims
The aim of the study was to evaluate the results of TKIs treatment in CML patients according to ELN2013 recommendations.
Methods
The prospective study included 71 adult patients with newly diagnosed CML in chronic phase. Baseline demographics characteristics: median age: 44 years (interquartile range (IQR) 31-57 years); male sex: 51% (n=36); Sokal score: high 23%, intermediate 27%, low 50%. Baseline treatment: imatinib (IM) 400 mg (n=63), nilotinib (NIL) 600 mg (n=7), dasatinib (DAS) 100 mg (n=1). The switching to another TKI or increasing doses was performed at 3,6,12 months or late in case of failure according to ELN2013 recommendations. Therapy was also changed in cases with no early molecular response (EMR) (BCR-ABL ≤10% at 3 months). Overall survival (OS), cumulative incidence of complete cytogenetic response (CCyR), major molecular response (MMR) and deep molecular response (MR4 or deeper) were evaluated (Intention-to-treat analysis).
Results
The failure was recorded in 14 (20%) cases (at 3 months n=6, at 6 months n=3, at 12 months n=2, after 12 months n=2, 1 patient died before 3 month assessment due to progression). Imatinib was a baseline treatment in all failures. Therapeutic options for failures: increasing dose of IM (600 mg) n=2, switching to DAS n=5, switching to NIL n=4, 2 patients are currently being examined before switching. In 6 cases switching or IM dose escalation was performed because of no EMR (out of failure) (NIL n=3, DAS n=1, IM600 mg n=2), other reasons (toxicity, late warning): n=4 (NIL n=2, DAS n=2). Median time to switching after failure detection was 67 days (IQR 36-103 days). Median follow-up: 19 months (IQR 12-31 months). Still alive: n=69; on IM treatment: n=44 (64%), on 2nd TKI: n=25 (36%). The 3-yars OS was 96%. Cumulative incidence of CCyR, MMR and MR4 was 98%, 86% and 73% respectively. Independent of switching, cumulative incidence of MR4 was higher in group with EMR than no EMR: 73% and 22%, respectively (p=0,03) (3 months landmark).
Conclusion
The principle of early therapy modification in patients with no optimal response according to ELN2013 response criteria allows to reach CCyR (the main surrogate endpoint for survival) in almost all cases. Early molecular response is a predictor of achieving deeper molecular response not depending on early switching approach.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Clinical outcome, Molecular response, Therapy
{{ help_message }}
{{filter}}


