EFFICACY AND SAFETY EVALUATION OF NILOTINIB AND DASATINIB (2G-TKI) ON FIRST LINE TREATMENT IN 73 PATIENTS WITH CML-CP OUTSIDE OF CLINICAL TRIALS. ANDALUSIAN CML REGISTRY (RALMC).
(Abstract release date: 05/19/16)
EHA Library. Puerta Puerta J. 06/09/16; 134720; PB1820

Mr. José Manuel Puerta Puerta
Contributions
Contributions
Abstract
Abstract: PB1820
Type: Publication Only
Background
Even though they were approved last June 2011 to be used on first line, it is not a common procedure to begin treatment of CML-CP with 2G-TKI, despite it has been demonstrated its efficacy and safety against imatinib on ENESTnd and Dasision clinical trials.
Aims
To describe the RALMC experience in terms of efficacy and safety of 2G-TKI on first line of treatment in CML-CP in real life evidence.
Methods
Descriptive analysis of 73 RALMC patients, treated from the outset in 18 hospitals of Andalusia (Spain) with nilotinib and dasatinib from June 2011 out of clinical trials. Results of BCR ABL are expressed in IS by means of GenXpert system. Responses are cataloged according to the 2013 ELN group guidelines. Toxicity of each treatment, overall survival (OS), failure free survival (FFS), event free survival (EFS) and progression free survival (PFS) were evaluated. Event was defined as death from any cause, progression to accelerated phase (AP) or blast crisis (BC), loss of CCgR or MMR and change of treatment for any reason.
Results
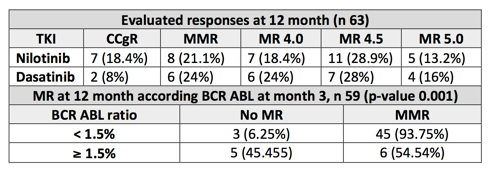
Median of 49 years of age (18-78) and median of follow-up of 38 months (3-56). 59% male, 41% female. 54% low, 26% intermediate, 20% high Sokal index. 79% low, 21% high Eutos score (n 63). Treatment of first line: nilotinib 46 (63%), dasatinib 27 (37%). Probability of achieving CCgR at 6 months was 100% (54 of 54 evaluated patients). Probability of achieving MMR at 12 months was 85,7% (54 of 63 evaluated patients) and MMR at 18 months 93,4% (57 of 61 evaluated patients). Depth of the responses are detailed in the table enclosed. No statistically significant differences are found between both treatments as they achieve MMR in months 12 and 18. In our patients treated with 2G-TKI in first line, probability to obtain BCR ABL ≤10% in month 3 (65 evaluated patients) was 100% and ≤1% of 77% (50 of 65 evaluated patients). In both branches of treatment, 77% of patients obtained rates of BCR ABL ≤1% (30 patients of 39 evaluated with nilotinib and 20 of 26 with dasatinib). Overall median value of BCR-ABL at 3 months was 0.16% (0.22% nilotinib, 0.15% dasatinib). Probability to achieve MMR in month 12 if BCR ABL in month 3 was <1.5% is of 94% as opposed to BCR ABL ≥1.5% which is 55% (p-value 0.001). Only one death is reported on the dasatinib branch (death related with CML). EFS with nilotinib was 85.5%, and 74.4% with dasatinib. FFS with nilotinib was 89.9% and 86.1% with dasatinib; there is no statistically significant differences between both treatments in terms of EFS and FFS (p-value 0.28 and 0.73 respectively). No patient of the series progresses to AP or BC. 12 treatment changes are carried out; 5 due to toxicity (7%): 2 with nilotinib (1 neutropenia, 1 dermatological toxicity) and 3 with dasatinib (1 pleural effusion, 1 thrombocytopenia, 1 ocular thrombosis). 7 treatment changes due to lack of efficacy (9.5%): 4 failures due to CCgR loss, 2 dasatinib and 2 nilotinib (mutational study was positive with nilotinib: 1 E308V and 1 T315l mutation), 2 fails due to MMR loss (1 dasatinib, 1 nilotinib) and 1 change from nilotinib to dasatinib in month 9 due to warning.
Conclusion
The use of nilotinib and dasatinib as first-line treatment is consolidated as an excellent therapeutic alternative to CML-CP. Showing with our series the efficacy and safety of 2G-TKI, with high rates of cytogenetic and molecular responses, deep and early, and low rates of toxicity carrying over treatment changes. The cutoff point of BCR ABL in month 3 of 1.5% could determines the optimal response in month 12 as soon as they achieve MMR.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Kinase inhibitor, Treatment
Type: Publication Only
Background
Even though they were approved last June 2011 to be used on first line, it is not a common procedure to begin treatment of CML-CP with 2G-TKI, despite it has been demonstrated its efficacy and safety against imatinib on ENESTnd and Dasision clinical trials.
Aims
To describe the RALMC experience in terms of efficacy and safety of 2G-TKI on first line of treatment in CML-CP in real life evidence.
Methods
Descriptive analysis of 73 RALMC patients, treated from the outset in 18 hospitals of Andalusia (Spain) with nilotinib and dasatinib from June 2011 out of clinical trials. Results of BCR ABL are expressed in IS by means of GenXpert system. Responses are cataloged according to the 2013 ELN group guidelines. Toxicity of each treatment, overall survival (OS), failure free survival (FFS), event free survival (EFS) and progression free survival (PFS) were evaluated. Event was defined as death from any cause, progression to accelerated phase (AP) or blast crisis (BC), loss of CCgR or MMR and change of treatment for any reason.
Results
Median of 49 years of age (18-78) and median of follow-up of 38 months (3-56). 59% male, 41% female. 54% low, 26% intermediate, 20% high Sokal index. 79% low, 21% high Eutos score (n 63). Treatment of first line: nilotinib 46 (63%), dasatinib 27 (37%). Probability of achieving CCgR at 6 months was 100% (54 of 54 evaluated patients). Probability of achieving MMR at 12 months was 85,7% (54 of 63 evaluated patients) and MMR at 18 months 93,4% (57 of 61 evaluated patients). Depth of the responses are detailed in the table enclosed. No statistically significant differences are found between both treatments as they achieve MMR in months 12 and 18. In our patients treated with 2G-TKI in first line, probability to obtain BCR ABL ≤10% in month 3 (65 evaluated patients) was 100% and ≤1% of 77% (50 of 65 evaluated patients). In both branches of treatment, 77% of patients obtained rates of BCR ABL ≤1% (30 patients of 39 evaluated with nilotinib and 20 of 26 with dasatinib). Overall median value of BCR-ABL at 3 months was 0.16% (0.22% nilotinib, 0.15% dasatinib). Probability to achieve MMR in month 12 if BCR ABL in month 3 was <1.5% is of 94% as opposed to BCR ABL ≥1.5% which is 55% (p-value 0.001). Only one death is reported on the dasatinib branch (death related with CML). EFS with nilotinib was 85.5%, and 74.4% with dasatinib. FFS with nilotinib was 89.9% and 86.1% with dasatinib; there is no statistically significant differences between both treatments in terms of EFS and FFS (p-value 0.28 and 0.73 respectively). No patient of the series progresses to AP or BC. 12 treatment changes are carried out; 5 due to toxicity (7%): 2 with nilotinib (1 neutropenia, 1 dermatological toxicity) and 3 with dasatinib (1 pleural effusion, 1 thrombocytopenia, 1 ocular thrombosis). 7 treatment changes due to lack of efficacy (9.5%): 4 failures due to CCgR loss, 2 dasatinib and 2 nilotinib (mutational study was positive with nilotinib: 1 E308V and 1 T315l mutation), 2 fails due to MMR loss (1 dasatinib, 1 nilotinib) and 1 change from nilotinib to dasatinib in month 9 due to warning.
Conclusion
The use of nilotinib and dasatinib as first-line treatment is consolidated as an excellent therapeutic alternative to CML-CP. Showing with our series the efficacy and safety of 2G-TKI, with high rates of cytogenetic and molecular responses, deep and early, and low rates of toxicity carrying over treatment changes. The cutoff point of BCR ABL in month 3 of 1.5% could determines the optimal response in month 12 as soon as they achieve MMR.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Kinase inhibitor, Treatment
Abstract: PB1820
Type: Publication Only
Background
Even though they were approved last June 2011 to be used on first line, it is not a common procedure to begin treatment of CML-CP with 2G-TKI, despite it has been demonstrated its efficacy and safety against imatinib on ENESTnd and Dasision clinical trials.
Aims
To describe the RALMC experience in terms of efficacy and safety of 2G-TKI on first line of treatment in CML-CP in real life evidence.
Methods
Descriptive analysis of 73 RALMC patients, treated from the outset in 18 hospitals of Andalusia (Spain) with nilotinib and dasatinib from June 2011 out of clinical trials. Results of BCR ABL are expressed in IS by means of GenXpert system. Responses are cataloged according to the 2013 ELN group guidelines. Toxicity of each treatment, overall survival (OS), failure free survival (FFS), event free survival (EFS) and progression free survival (PFS) were evaluated. Event was defined as death from any cause, progression to accelerated phase (AP) or blast crisis (BC), loss of CCgR or MMR and change of treatment for any reason.
Results
Median of 49 years of age (18-78) and median of follow-up of 38 months (3-56). 59% male, 41% female. 54% low, 26% intermediate, 20% high Sokal index. 79% low, 21% high Eutos score (n 63). Treatment of first line: nilotinib 46 (63%), dasatinib 27 (37%). Probability of achieving CCgR at 6 months was 100% (54 of 54 evaluated patients). Probability of achieving MMR at 12 months was 85,7% (54 of 63 evaluated patients) and MMR at 18 months 93,4% (57 of 61 evaluated patients). Depth of the responses are detailed in the table enclosed. No statistically significant differences are found between both treatments as they achieve MMR in months 12 and 18. In our patients treated with 2G-TKI in first line, probability to obtain BCR ABL ≤10% in month 3 (65 evaluated patients) was 100% and ≤1% of 77% (50 of 65 evaluated patients). In both branches of treatment, 77% of patients obtained rates of BCR ABL ≤1% (30 patients of 39 evaluated with nilotinib and 20 of 26 with dasatinib). Overall median value of BCR-ABL at 3 months was 0.16% (0.22% nilotinib, 0.15% dasatinib). Probability to achieve MMR in month 12 if BCR ABL in month 3 was <1.5% is of 94% as opposed to BCR ABL ≥1.5% which is 55% (p-value 0.001). Only one death is reported on the dasatinib branch (death related with CML). EFS with nilotinib was 85.5%, and 74.4% with dasatinib. FFS with nilotinib was 89.9% and 86.1% with dasatinib; there is no statistically significant differences between both treatments in terms of EFS and FFS (p-value 0.28 and 0.73 respectively). No patient of the series progresses to AP or BC. 12 treatment changes are carried out; 5 due to toxicity (7%): 2 with nilotinib (1 neutropenia, 1 dermatological toxicity) and 3 with dasatinib (1 pleural effusion, 1 thrombocytopenia, 1 ocular thrombosis). 7 treatment changes due to lack of efficacy (9.5%): 4 failures due to CCgR loss, 2 dasatinib and 2 nilotinib (mutational study was positive with nilotinib: 1 E308V and 1 T315l mutation), 2 fails due to MMR loss (1 dasatinib, 1 nilotinib) and 1 change from nilotinib to dasatinib in month 9 due to warning.
Conclusion
The use of nilotinib and dasatinib as first-line treatment is consolidated as an excellent therapeutic alternative to CML-CP. Showing with our series the efficacy and safety of 2G-TKI, with high rates of cytogenetic and molecular responses, deep and early, and low rates of toxicity carrying over treatment changes. The cutoff point of BCR ABL in month 3 of 1.5% could determines the optimal response in month 12 as soon as they achieve MMR.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Kinase inhibitor, Treatment
Type: Publication Only
Background
Even though they were approved last June 2011 to be used on first line, it is not a common procedure to begin treatment of CML-CP with 2G-TKI, despite it has been demonstrated its efficacy and safety against imatinib on ENESTnd and Dasision clinical trials.
Aims
To describe the RALMC experience in terms of efficacy and safety of 2G-TKI on first line of treatment in CML-CP in real life evidence.
Methods
Descriptive analysis of 73 RALMC patients, treated from the outset in 18 hospitals of Andalusia (Spain) with nilotinib and dasatinib from June 2011 out of clinical trials. Results of BCR ABL are expressed in IS by means of GenXpert system. Responses are cataloged according to the 2013 ELN group guidelines. Toxicity of each treatment, overall survival (OS), failure free survival (FFS), event free survival (EFS) and progression free survival (PFS) were evaluated. Event was defined as death from any cause, progression to accelerated phase (AP) or blast crisis (BC), loss of CCgR or MMR and change of treatment for any reason.
Results
Median of 49 years of age (18-78) and median of follow-up of 38 months (3-56). 59% male, 41% female. 54% low, 26% intermediate, 20% high Sokal index. 79% low, 21% high Eutos score (n 63). Treatment of first line: nilotinib 46 (63%), dasatinib 27 (37%). Probability of achieving CCgR at 6 months was 100% (54 of 54 evaluated patients). Probability of achieving MMR at 12 months was 85,7% (54 of 63 evaluated patients) and MMR at 18 months 93,4% (57 of 61 evaluated patients). Depth of the responses are detailed in the table enclosed. No statistically significant differences are found between both treatments as they achieve MMR in months 12 and 18. In our patients treated with 2G-TKI in first line, probability to obtain BCR ABL ≤10% in month 3 (65 evaluated patients) was 100% and ≤1% of 77% (50 of 65 evaluated patients). In both branches of treatment, 77% of patients obtained rates of BCR ABL ≤1% (30 patients of 39 evaluated with nilotinib and 20 of 26 with dasatinib). Overall median value of BCR-ABL at 3 months was 0.16% (0.22% nilotinib, 0.15% dasatinib). Probability to achieve MMR in month 12 if BCR ABL in month 3 was <1.5% is of 94% as opposed to BCR ABL ≥1.5% which is 55% (p-value 0.001). Only one death is reported on the dasatinib branch (death related with CML). EFS with nilotinib was 85.5%, and 74.4% with dasatinib. FFS with nilotinib was 89.9% and 86.1% with dasatinib; there is no statistically significant differences between both treatments in terms of EFS and FFS (p-value 0.28 and 0.73 respectively). No patient of the series progresses to AP or BC. 12 treatment changes are carried out; 5 due to toxicity (7%): 2 with nilotinib (1 neutropenia, 1 dermatological toxicity) and 3 with dasatinib (1 pleural effusion, 1 thrombocytopenia, 1 ocular thrombosis). 7 treatment changes due to lack of efficacy (9.5%): 4 failures due to CCgR loss, 2 dasatinib and 2 nilotinib (mutational study was positive with nilotinib: 1 E308V and 1 T315l mutation), 2 fails due to MMR loss (1 dasatinib, 1 nilotinib) and 1 change from nilotinib to dasatinib in month 9 due to warning.
Conclusion
The use of nilotinib and dasatinib as first-line treatment is consolidated as an excellent therapeutic alternative to CML-CP. Showing with our series the efficacy and safety of 2G-TKI, with high rates of cytogenetic and molecular responses, deep and early, and low rates of toxicity carrying over treatment changes. The cutoff point of BCR ABL in month 3 of 1.5% could determines the optimal response in month 12 as soon as they achieve MMR.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Kinase inhibitor, Treatment
{{ help_message }}
{{filter}}


