PREDICTIVE FACTORS OF STABLE DEEP MOLECULAR RESPONSE IN CHRONIC PHASE CML PATIENTS TREATED WITH DASATINIB OR NILOTINIB AFTER IMATINIB FAILURE
(Abstract release date: 05/19/16)
EHA Library. Bonifacio M. 06/09/16; 134718; PB1818

Dr. Massimiliano Bonifacio
Contributions
Contributions
Abstract
Abstract: PB1818
Type: Publication Only
Background
Front-line treatment of newly diagnosed Chronic Myeloid Leukemia (CML) patients with 2nd generation tyrosine kinase inhibitors (2G-TKIs) dasatinib (DAS) or nilotinib (NIL) resulted in higher rates of deep molecular response (MR) as compared to imatinib. Very little is known about rates and stability of deep MR when 2G-TKIs are used after imatinib failure.
Aims
To assess the probability and predictors of stable deep MR in ≥2nd line of treatment.
Methods
We restrospectively analyzed 127 chronic phase CML patients treated with imatinib 400 mg daily as first-line therapy and then switched to 2G-TKIs for resistance or intolerance. Patients progressing to advanced phases before switch were excluded. Deep molecular response (MR4) was defined as BCL-ABRIS ratio ≤0.01% or undetectable disease with ≥10,000 ABL copies. Patients with MR4 lasting ≥ 2 years, ongoing at the last contact, and with at least a Q-PCR test every 6 months were defined as stable MR4. Patients with any sample >0.01% BCR-ABLIS after the achievement of MR4 were defined as unstable MR4. Age, sex, Sokal and EUTOS risk score, type of BCR-ABL transcript, duration of imatinib, reason for switch to 2G-TKIs and early molecular response to 2G-TKIs have been examined for the association with stable MR4. Frequencies were compared by Fisher’s exact test. Univariate and multivariate regression analysis were performed using the competing risk model of Fine and Gray.
Results
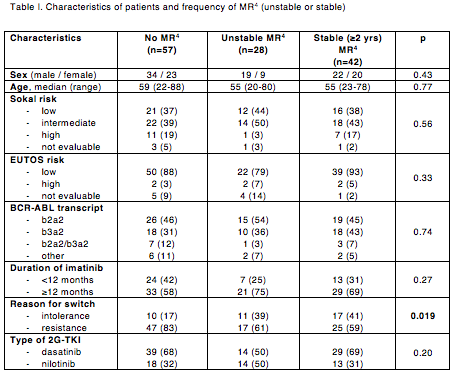
Median age at diagnosis was 55 years (range 20-88) and median duration of imatinib treatment was 19 months (range 1-115). Patients resistant (n=89; 70%) or intolerant (n=38; 30%) to imatinib were switched to DAS (n=82; 64.5%) or NIL (n=45; 35.5%). Thirty-six patients were resistant or intolerant to their first 2G-TKI and were switched to 3rd-line NIL (n=20), DAS (n=12), bosutinib (n=2), or ponatinib (n=2). At a median follow-up of 52 months after switch to 2G-TKI (range 6-126), best deep MR to 2G-TKI was: no MR4 in 57 patients (45%), unstable MR4 in 28 patients (22%; 24 with 2nd line and 4 with 3rd line treatment), and stable MR4 in 42 patients (33%; 37 with 2nd line and 5 with 3rd line treatment). Five-year cumulative incidence of stable MR4 was 28.7% (95%CI: 18.9-37.3%). Age, sex, risk scores at diagnosis, type of BCR-ABL transcript, duration of imatinib and type of 2G-TKI were similar between patients with or without stable MR4 (Table I). Predictors of stable MR4 were reason for switch to 2G-TKI (intolerance vs resistance HR 0.41, 95%CI: 0.22-0.79; p=0.007) and 3-month BCR-ABL level after 2G-TKI start (≤10%IS vs >10%IS HR 0.08, 95%CI: 0.01-0.59; p=0.01). Three stable MR4 patients attempted discontinuation and are presently in treatment-free remission phase at 2, 22 and 27 months after 2G-TKI stop, respectively.
Conclusion
In this retrospective, real-life experience, long-term use of 2G-TKIs in ≥2nd line of treatment after imatinib failure resulted in more than half of patients achieving the “safe haven” of deep MR, with around 60% of them in stable MR4, a prerequisite for discontinuing treatment.
Session topic: E-poster
Keyword(s): Molecular response, Prediction, Tyrosine kinase inhibitor
Type: Publication Only
Background
Front-line treatment of newly diagnosed Chronic Myeloid Leukemia (CML) patients with 2nd generation tyrosine kinase inhibitors (2G-TKIs) dasatinib (DAS) or nilotinib (NIL) resulted in higher rates of deep molecular response (MR) as compared to imatinib. Very little is known about rates and stability of deep MR when 2G-TKIs are used after imatinib failure.
Aims
To assess the probability and predictors of stable deep MR in ≥2nd line of treatment.
Methods
We restrospectively analyzed 127 chronic phase CML patients treated with imatinib 400 mg daily as first-line therapy and then switched to 2G-TKIs for resistance or intolerance. Patients progressing to advanced phases before switch were excluded. Deep molecular response (MR4) was defined as BCL-ABRIS ratio ≤0.01% or undetectable disease with ≥10,000 ABL copies. Patients with MR4 lasting ≥ 2 years, ongoing at the last contact, and with at least a Q-PCR test every 6 months were defined as stable MR4. Patients with any sample >0.01% BCR-ABLIS after the achievement of MR4 were defined as unstable MR4. Age, sex, Sokal and EUTOS risk score, type of BCR-ABL transcript, duration of imatinib, reason for switch to 2G-TKIs and early molecular response to 2G-TKIs have been examined for the association with stable MR4. Frequencies were compared by Fisher’s exact test. Univariate and multivariate regression analysis were performed using the competing risk model of Fine and Gray.
Results
Median age at diagnosis was 55 years (range 20-88) and median duration of imatinib treatment was 19 months (range 1-115). Patients resistant (n=89; 70%) or intolerant (n=38; 30%) to imatinib were switched to DAS (n=82; 64.5%) or NIL (n=45; 35.5%). Thirty-six patients were resistant or intolerant to their first 2G-TKI and were switched to 3rd-line NIL (n=20), DAS (n=12), bosutinib (n=2), or ponatinib (n=2). At a median follow-up of 52 months after switch to 2G-TKI (range 6-126), best deep MR to 2G-TKI was: no MR4 in 57 patients (45%), unstable MR4 in 28 patients (22%; 24 with 2nd line and 4 with 3rd line treatment), and stable MR4 in 42 patients (33%; 37 with 2nd line and 5 with 3rd line treatment). Five-year cumulative incidence of stable MR4 was 28.7% (95%CI: 18.9-37.3%). Age, sex, risk scores at diagnosis, type of BCR-ABL transcript, duration of imatinib and type of 2G-TKI were similar between patients with or without stable MR4 (Table I). Predictors of stable MR4 were reason for switch to 2G-TKI (intolerance vs resistance HR 0.41, 95%CI: 0.22-0.79; p=0.007) and 3-month BCR-ABL level after 2G-TKI start (≤10%IS vs >10%IS HR 0.08, 95%CI: 0.01-0.59; p=0.01). Three stable MR4 patients attempted discontinuation and are presently in treatment-free remission phase at 2, 22 and 27 months after 2G-TKI stop, respectively.
Conclusion
In this retrospective, real-life experience, long-term use of 2G-TKIs in ≥2nd line of treatment after imatinib failure resulted in more than half of patients achieving the “safe haven” of deep MR, with around 60% of them in stable MR4, a prerequisite for discontinuing treatment.

Session topic: E-poster
Keyword(s): Molecular response, Prediction, Tyrosine kinase inhibitor
Abstract: PB1818
Type: Publication Only
Background
Front-line treatment of newly diagnosed Chronic Myeloid Leukemia (CML) patients with 2nd generation tyrosine kinase inhibitors (2G-TKIs) dasatinib (DAS) or nilotinib (NIL) resulted in higher rates of deep molecular response (MR) as compared to imatinib. Very little is known about rates and stability of deep MR when 2G-TKIs are used after imatinib failure.
Aims
To assess the probability and predictors of stable deep MR in ≥2nd line of treatment.
Methods
We restrospectively analyzed 127 chronic phase CML patients treated with imatinib 400 mg daily as first-line therapy and then switched to 2G-TKIs for resistance or intolerance. Patients progressing to advanced phases before switch were excluded. Deep molecular response (MR4) was defined as BCL-ABRIS ratio ≤0.01% or undetectable disease with ≥10,000 ABL copies. Patients with MR4 lasting ≥ 2 years, ongoing at the last contact, and with at least a Q-PCR test every 6 months were defined as stable MR4. Patients with any sample >0.01% BCR-ABLIS after the achievement of MR4 were defined as unstable MR4. Age, sex, Sokal and EUTOS risk score, type of BCR-ABL transcript, duration of imatinib, reason for switch to 2G-TKIs and early molecular response to 2G-TKIs have been examined for the association with stable MR4. Frequencies were compared by Fisher’s exact test. Univariate and multivariate regression analysis were performed using the competing risk model of Fine and Gray.
Results
Median age at diagnosis was 55 years (range 20-88) and median duration of imatinib treatment was 19 months (range 1-115). Patients resistant (n=89; 70%) or intolerant (n=38; 30%) to imatinib were switched to DAS (n=82; 64.5%) or NIL (n=45; 35.5%). Thirty-six patients were resistant or intolerant to their first 2G-TKI and were switched to 3rd-line NIL (n=20), DAS (n=12), bosutinib (n=2), or ponatinib (n=2). At a median follow-up of 52 months after switch to 2G-TKI (range 6-126), best deep MR to 2G-TKI was: no MR4 in 57 patients (45%), unstable MR4 in 28 patients (22%; 24 with 2nd line and 4 with 3rd line treatment), and stable MR4 in 42 patients (33%; 37 with 2nd line and 5 with 3rd line treatment). Five-year cumulative incidence of stable MR4 was 28.7% (95%CI: 18.9-37.3%). Age, sex, risk scores at diagnosis, type of BCR-ABL transcript, duration of imatinib and type of 2G-TKI were similar between patients with or without stable MR4 (Table I). Predictors of stable MR4 were reason for switch to 2G-TKI (intolerance vs resistance HR 0.41, 95%CI: 0.22-0.79; p=0.007) and 3-month BCR-ABL level after 2G-TKI start (≤10%IS vs >10%IS HR 0.08, 95%CI: 0.01-0.59; p=0.01). Three stable MR4 patients attempted discontinuation and are presently in treatment-free remission phase at 2, 22 and 27 months after 2G-TKI stop, respectively.
Conclusion
In this retrospective, real-life experience, long-term use of 2G-TKIs in ≥2nd line of treatment after imatinib failure resulted in more than half of patients achieving the “safe haven” of deep MR, with around 60% of them in stable MR4, a prerequisite for discontinuing treatment.

Session topic: E-poster
Keyword(s): Molecular response, Prediction, Tyrosine kinase inhibitor
Type: Publication Only
Background
Front-line treatment of newly diagnosed Chronic Myeloid Leukemia (CML) patients with 2nd generation tyrosine kinase inhibitors (2G-TKIs) dasatinib (DAS) or nilotinib (NIL) resulted in higher rates of deep molecular response (MR) as compared to imatinib. Very little is known about rates and stability of deep MR when 2G-TKIs are used after imatinib failure.
Aims
To assess the probability and predictors of stable deep MR in ≥2nd line of treatment.
Methods
We restrospectively analyzed 127 chronic phase CML patients treated with imatinib 400 mg daily as first-line therapy and then switched to 2G-TKIs for resistance or intolerance. Patients progressing to advanced phases before switch were excluded. Deep molecular response (MR4) was defined as BCL-ABRIS ratio ≤0.01% or undetectable disease with ≥10,000 ABL copies. Patients with MR4 lasting ≥ 2 years, ongoing at the last contact, and with at least a Q-PCR test every 6 months were defined as stable MR4. Patients with any sample >0.01% BCR-ABLIS after the achievement of MR4 were defined as unstable MR4. Age, sex, Sokal and EUTOS risk score, type of BCR-ABL transcript, duration of imatinib, reason for switch to 2G-TKIs and early molecular response to 2G-TKIs have been examined for the association with stable MR4. Frequencies were compared by Fisher’s exact test. Univariate and multivariate regression analysis were performed using the competing risk model of Fine and Gray.
Results
Median age at diagnosis was 55 years (range 20-88) and median duration of imatinib treatment was 19 months (range 1-115). Patients resistant (n=89; 70%) or intolerant (n=38; 30%) to imatinib were switched to DAS (n=82; 64.5%) or NIL (n=45; 35.5%). Thirty-six patients were resistant or intolerant to their first 2G-TKI and were switched to 3rd-line NIL (n=20), DAS (n=12), bosutinib (n=2), or ponatinib (n=2). At a median follow-up of 52 months after switch to 2G-TKI (range 6-126), best deep MR to 2G-TKI was: no MR4 in 57 patients (45%), unstable MR4 in 28 patients (22%; 24 with 2nd line and 4 with 3rd line treatment), and stable MR4 in 42 patients (33%; 37 with 2nd line and 5 with 3rd line treatment). Five-year cumulative incidence of stable MR4 was 28.7% (95%CI: 18.9-37.3%). Age, sex, risk scores at diagnosis, type of BCR-ABL transcript, duration of imatinib and type of 2G-TKI were similar between patients with or without stable MR4 (Table I). Predictors of stable MR4 were reason for switch to 2G-TKI (intolerance vs resistance HR 0.41, 95%CI: 0.22-0.79; p=0.007) and 3-month BCR-ABL level after 2G-TKI start (≤10%IS vs >10%IS HR 0.08, 95%CI: 0.01-0.59; p=0.01). Three stable MR4 patients attempted discontinuation and are presently in treatment-free remission phase at 2, 22 and 27 months after 2G-TKI stop, respectively.
Conclusion
In this retrospective, real-life experience, long-term use of 2G-TKIs in ≥2nd line of treatment after imatinib failure resulted in more than half of patients achieving the “safe haven” of deep MR, with around 60% of them in stable MR4, a prerequisite for discontinuing treatment.

Session topic: E-poster
Keyword(s): Molecular response, Prediction, Tyrosine kinase inhibitor
{{ help_message }}
{{filter}}


