REDISTRIBUTION PATTERN AND ASSESSMENT OF EARLY RESPONSE TO IBRUTINIB IN CLL BY TOTAL TUMOR MASS SCORE (TTM) - KROHEM CLL2 STUDY
(Abstract release date: 05/19/16)
EHA Library. Jaksic O. 06/09/16; 134688; PB1788

Prof. Dr. Ozren Jaksic
Contributions
Contributions
Abstract
Abstract: PB1788
Type: Publication Only
Background
Ibrutinib is Bruton’s tyrosine kinase inhibitor with significant efficacy in CLL. Ibrutinib monotherapy usually leads to redistribution of tumor mass from lymphoid organs to peripheral blood before eventual response in all compartments. This leads to certain problems in response assessment by iwCLL criteria requiring several modifications. Total Tumor Mass score (TTM) (Jaksic, BJH 1980) is a simple quantitative clinical parameter for evaluation of tumor mass in all relevant lymphoid compartments in CLL (PB+BM, LN and spleen) that was successfully used in CLL trials by several international cooperative groups (EORTC, IGCI) in alkylator and purine analog era. It is helpful in routine clinical practice for monitoring of total tumor load, progression and/or response to treatment. Due to its characteristics TTM may overcome problems caused by significant redistribution during TKI treatment (Jaksic, BJH 2014).
Aims
To evaluate usefulness of TTM in evaluation of response and redistribution during ibrutinib treatment in CLL patients in a real life setting.
Methods
This is an observational study from Croatian cooperative group for hematological malignancies (KROHEM) were data from patients included in ibrutinib NPP (420 mg daily) were collected on national level. Thirty patients with relapsed / refractory B-CLL were included, 24 males and 6 females, median age 65.5 years (50-83) and median 2 prior lines of therapy (1-6). There were 4 pts with del 17p and 6 with del 11q (out of 24 with data). Baseline routine evaluation was done before ibrutinib treatment. Clinical and laboratory assessments of tumor mass were collected at start and on months 1, 3, 6, 9 and 12. Bone marrow assessment, US and MSCT scans were performed when clinically indicated. Response was assessed by modified iwCLL criteria (Hallek, Blood 2008, 2012, 2013) and by TTM criteria (Jaksic, BJH 2014).TTM scoring system: TM1= √Lymphocytes (×109/l), TM2=largest palpable lymph node (cm), TM3=spleen below left costal margin (cm).TTM = TM1+TM2+TM3, TTM-D= TM1/TTM (percentage of tumor mass in leukemic compartment).∆TTM – response/progression, ∆TTM-D - redistribution
Results
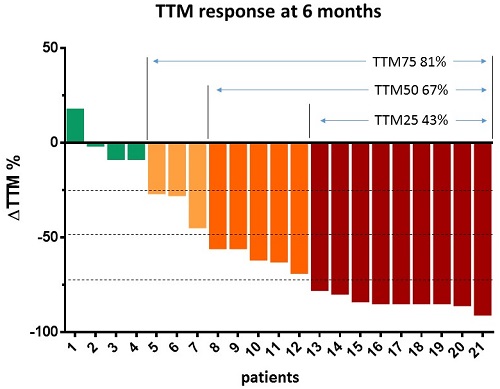
At 6th month 81% of patients responded with tumor mass (TTM) reduction > 25%; 67% of patients responded with tumor mass reduction >50% and 43% of patients responded with tumor mass reduction > 75%. Modified iwCLL response correlated well with TTM response criteria on months 3, 6 and 9 (Spearman correlation 0.538, p<0.001; 0.762, p<0.001; 0.739, p<0,009 respectively), but not in month 1 (Spearman correlation 0.175, p=0.356). At month 6, there were 13/21 patients in PR and PR+PR-Ly 19/21 (iwCLL) compared to 14/21 (TTM reduced > 50%) and 17/21 (TTM reduced >25%) respectively. Lymphocytosis changed from baseline = 1.0 to months 1, 3, 6 and 9 (median 2.60; 1.95; 0.67 and 0.51 respectively) taking into account all cases. However, while this describes a typical increase, actually no increase of lymphocytosis at month 1 was observed in 30% of patients. Neither multiple stepwise regression found any statistically significant correlation with baseline predictors, nor were patients with increase of lymphocytosis at month 1 significantly different from typical responders in distribution of baseline predictors according to Mann Whitney U test. Tumor distribution (TTM-D, median) rose from 0.47 (baseline) to 0.84 in month 1, and to 1.0 from months 3 on. Multiple stepwise regression with response at month 6 as dependent variable and 10 predictor variables at baseline: age, disease duration, previous lines of therapy, lymphocytosis, lymph node size, spleen size, TTM, TTM-D, hemoglobin and platelets identified in the model only TTM at baseline as significant negative predictor of response (p=0.001).
Conclusion
TTM is a simple and useful parameter successfully applied in ibrutinib treated patients in routine clinical practice for response assessment and follow-up. It is highly correlated with modified iwCLL criteria after 3rd month. It is more robust then iwCLL criteria because it incorporates and quantify tumor redistribution and therefore can be applied regardless of therapy used without specific modifications.
Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Kinase inhibitor
Type: Publication Only
Background
Ibrutinib is Bruton’s tyrosine kinase inhibitor with significant efficacy in CLL. Ibrutinib monotherapy usually leads to redistribution of tumor mass from lymphoid organs to peripheral blood before eventual response in all compartments. This leads to certain problems in response assessment by iwCLL criteria requiring several modifications. Total Tumor Mass score (TTM) (Jaksic, BJH 1980) is a simple quantitative clinical parameter for evaluation of tumor mass in all relevant lymphoid compartments in CLL (PB+BM, LN and spleen) that was successfully used in CLL trials by several international cooperative groups (EORTC, IGCI) in alkylator and purine analog era. It is helpful in routine clinical practice for monitoring of total tumor load, progression and/or response to treatment. Due to its characteristics TTM may overcome problems caused by significant redistribution during TKI treatment (Jaksic, BJH 2014).
Aims
To evaluate usefulness of TTM in evaluation of response and redistribution during ibrutinib treatment in CLL patients in a real life setting.
Methods
This is an observational study from Croatian cooperative group for hematological malignancies (KROHEM) were data from patients included in ibrutinib NPP (420 mg daily) were collected on national level. Thirty patients with relapsed / refractory B-CLL were included, 24 males and 6 females, median age 65.5 years (50-83) and median 2 prior lines of therapy (1-6). There were 4 pts with del 17p and 6 with del 11q (out of 24 with data). Baseline routine evaluation was done before ibrutinib treatment. Clinical and laboratory assessments of tumor mass were collected at start and on months 1, 3, 6, 9 and 12. Bone marrow assessment, US and MSCT scans were performed when clinically indicated. Response was assessed by modified iwCLL criteria (Hallek, Blood 2008, 2012, 2013) and by TTM criteria (Jaksic, BJH 2014).TTM scoring system: TM1= √Lymphocytes (×109/l), TM2=largest palpable lymph node (cm), TM3=spleen below left costal margin (cm).TTM = TM1+TM2+TM3, TTM-D= TM1/TTM (percentage of tumor mass in leukemic compartment).∆TTM – response/progression, ∆TTM-D - redistribution
Results
At 6th month 81% of patients responded with tumor mass (TTM) reduction > 25%; 67% of patients responded with tumor mass reduction >50% and 43% of patients responded with tumor mass reduction > 75%. Modified iwCLL response correlated well with TTM response criteria on months 3, 6 and 9 (Spearman correlation 0.538, p<0.001; 0.762, p<0.001; 0.739, p<0,009 respectively), but not in month 1 (Spearman correlation 0.175, p=0.356). At month 6, there were 13/21 patients in PR and PR+PR-Ly 19/21 (iwCLL) compared to 14/21 (TTM reduced > 50%) and 17/21 (TTM reduced >25%) respectively. Lymphocytosis changed from baseline = 1.0 to months 1, 3, 6 and 9 (median 2.60; 1.95; 0.67 and 0.51 respectively) taking into account all cases. However, while this describes a typical increase, actually no increase of lymphocytosis at month 1 was observed in 30% of patients. Neither multiple stepwise regression found any statistically significant correlation with baseline predictors, nor were patients with increase of lymphocytosis at month 1 significantly different from typical responders in distribution of baseline predictors according to Mann Whitney U test. Tumor distribution (TTM-D, median) rose from 0.47 (baseline) to 0.84 in month 1, and to 1.0 from months 3 on. Multiple stepwise regression with response at month 6 as dependent variable and 10 predictor variables at baseline: age, disease duration, previous lines of therapy, lymphocytosis, lymph node size, spleen size, TTM, TTM-D, hemoglobin and platelets identified in the model only TTM at baseline as significant negative predictor of response (p=0.001).
Conclusion
TTM is a simple and useful parameter successfully applied in ibrutinib treated patients in routine clinical practice for response assessment and follow-up. It is highly correlated with modified iwCLL criteria after 3rd month. It is more robust then iwCLL criteria because it incorporates and quantify tumor redistribution and therefore can be applied regardless of therapy used without specific modifications.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Kinase inhibitor
Abstract: PB1788
Type: Publication Only
Background
Ibrutinib is Bruton’s tyrosine kinase inhibitor with significant efficacy in CLL. Ibrutinib monotherapy usually leads to redistribution of tumor mass from lymphoid organs to peripheral blood before eventual response in all compartments. This leads to certain problems in response assessment by iwCLL criteria requiring several modifications. Total Tumor Mass score (TTM) (Jaksic, BJH 1980) is a simple quantitative clinical parameter for evaluation of tumor mass in all relevant lymphoid compartments in CLL (PB+BM, LN and spleen) that was successfully used in CLL trials by several international cooperative groups (EORTC, IGCI) in alkylator and purine analog era. It is helpful in routine clinical practice for monitoring of total tumor load, progression and/or response to treatment. Due to its characteristics TTM may overcome problems caused by significant redistribution during TKI treatment (Jaksic, BJH 2014).
Aims
To evaluate usefulness of TTM in evaluation of response and redistribution during ibrutinib treatment in CLL patients in a real life setting.
Methods
This is an observational study from Croatian cooperative group for hematological malignancies (KROHEM) were data from patients included in ibrutinib NPP (420 mg daily) were collected on national level. Thirty patients with relapsed / refractory B-CLL were included, 24 males and 6 females, median age 65.5 years (50-83) and median 2 prior lines of therapy (1-6). There were 4 pts with del 17p and 6 with del 11q (out of 24 with data). Baseline routine evaluation was done before ibrutinib treatment. Clinical and laboratory assessments of tumor mass were collected at start and on months 1, 3, 6, 9 and 12. Bone marrow assessment, US and MSCT scans were performed when clinically indicated. Response was assessed by modified iwCLL criteria (Hallek, Blood 2008, 2012, 2013) and by TTM criteria (Jaksic, BJH 2014).TTM scoring system: TM1= √Lymphocytes (×109/l), TM2=largest palpable lymph node (cm), TM3=spleen below left costal margin (cm).TTM = TM1+TM2+TM3, TTM-D= TM1/TTM (percentage of tumor mass in leukemic compartment).∆TTM – response/progression, ∆TTM-D - redistribution
Results
At 6th month 81% of patients responded with tumor mass (TTM) reduction > 25%; 67% of patients responded with tumor mass reduction >50% and 43% of patients responded with tumor mass reduction > 75%. Modified iwCLL response correlated well with TTM response criteria on months 3, 6 and 9 (Spearman correlation 0.538, p<0.001; 0.762, p<0.001; 0.739, p<0,009 respectively), but not in month 1 (Spearman correlation 0.175, p=0.356). At month 6, there were 13/21 patients in PR and PR+PR-Ly 19/21 (iwCLL) compared to 14/21 (TTM reduced > 50%) and 17/21 (TTM reduced >25%) respectively. Lymphocytosis changed from baseline = 1.0 to months 1, 3, 6 and 9 (median 2.60; 1.95; 0.67 and 0.51 respectively) taking into account all cases. However, while this describes a typical increase, actually no increase of lymphocytosis at month 1 was observed in 30% of patients. Neither multiple stepwise regression found any statistically significant correlation with baseline predictors, nor were patients with increase of lymphocytosis at month 1 significantly different from typical responders in distribution of baseline predictors according to Mann Whitney U test. Tumor distribution (TTM-D, median) rose from 0.47 (baseline) to 0.84 in month 1, and to 1.0 from months 3 on. Multiple stepwise regression with response at month 6 as dependent variable and 10 predictor variables at baseline: age, disease duration, previous lines of therapy, lymphocytosis, lymph node size, spleen size, TTM, TTM-D, hemoglobin and platelets identified in the model only TTM at baseline as significant negative predictor of response (p=0.001).
Conclusion
TTM is a simple and useful parameter successfully applied in ibrutinib treated patients in routine clinical practice for response assessment and follow-up. It is highly correlated with modified iwCLL criteria after 3rd month. It is more robust then iwCLL criteria because it incorporates and quantify tumor redistribution and therefore can be applied regardless of therapy used without specific modifications.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Kinase inhibitor
Type: Publication Only
Background
Ibrutinib is Bruton’s tyrosine kinase inhibitor with significant efficacy in CLL. Ibrutinib monotherapy usually leads to redistribution of tumor mass from lymphoid organs to peripheral blood before eventual response in all compartments. This leads to certain problems in response assessment by iwCLL criteria requiring several modifications. Total Tumor Mass score (TTM) (Jaksic, BJH 1980) is a simple quantitative clinical parameter for evaluation of tumor mass in all relevant lymphoid compartments in CLL (PB+BM, LN and spleen) that was successfully used in CLL trials by several international cooperative groups (EORTC, IGCI) in alkylator and purine analog era. It is helpful in routine clinical practice for monitoring of total tumor load, progression and/or response to treatment. Due to its characteristics TTM may overcome problems caused by significant redistribution during TKI treatment (Jaksic, BJH 2014).
Aims
To evaluate usefulness of TTM in evaluation of response and redistribution during ibrutinib treatment in CLL patients in a real life setting.
Methods
This is an observational study from Croatian cooperative group for hematological malignancies (KROHEM) were data from patients included in ibrutinib NPP (420 mg daily) were collected on national level. Thirty patients with relapsed / refractory B-CLL were included, 24 males and 6 females, median age 65.5 years (50-83) and median 2 prior lines of therapy (1-6). There were 4 pts with del 17p and 6 with del 11q (out of 24 with data). Baseline routine evaluation was done before ibrutinib treatment. Clinical and laboratory assessments of tumor mass were collected at start and on months 1, 3, 6, 9 and 12. Bone marrow assessment, US and MSCT scans were performed when clinically indicated. Response was assessed by modified iwCLL criteria (Hallek, Blood 2008, 2012, 2013) and by TTM criteria (Jaksic, BJH 2014).TTM scoring system: TM1= √Lymphocytes (×109/l), TM2=largest palpable lymph node (cm), TM3=spleen below left costal margin (cm).TTM = TM1+TM2+TM3, TTM-D= TM1/TTM (percentage of tumor mass in leukemic compartment).∆TTM – response/progression, ∆TTM-D - redistribution
Results
At 6th month 81% of patients responded with tumor mass (TTM) reduction > 25%; 67% of patients responded with tumor mass reduction >50% and 43% of patients responded with tumor mass reduction > 75%. Modified iwCLL response correlated well with TTM response criteria on months 3, 6 and 9 (Spearman correlation 0.538, p<0.001; 0.762, p<0.001; 0.739, p<0,009 respectively), but not in month 1 (Spearman correlation 0.175, p=0.356). At month 6, there were 13/21 patients in PR and PR+PR-Ly 19/21 (iwCLL) compared to 14/21 (TTM reduced > 50%) and 17/21 (TTM reduced >25%) respectively. Lymphocytosis changed from baseline = 1.0 to months 1, 3, 6 and 9 (median 2.60; 1.95; 0.67 and 0.51 respectively) taking into account all cases. However, while this describes a typical increase, actually no increase of lymphocytosis at month 1 was observed in 30% of patients. Neither multiple stepwise regression found any statistically significant correlation with baseline predictors, nor were patients with increase of lymphocytosis at month 1 significantly different from typical responders in distribution of baseline predictors according to Mann Whitney U test. Tumor distribution (TTM-D, median) rose from 0.47 (baseline) to 0.84 in month 1, and to 1.0 from months 3 on. Multiple stepwise regression with response at month 6 as dependent variable and 10 predictor variables at baseline: age, disease duration, previous lines of therapy, lymphocytosis, lymph node size, spleen size, TTM, TTM-D, hemoglobin and platelets identified in the model only TTM at baseline as significant negative predictor of response (p=0.001).
Conclusion
TTM is a simple and useful parameter successfully applied in ibrutinib treated patients in routine clinical practice for response assessment and follow-up. It is highly correlated with modified iwCLL criteria after 3rd month. It is more robust then iwCLL criteria because it incorporates and quantify tumor redistribution and therefore can be applied regardless of therapy used without specific modifications.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Kinase inhibitor
{{ help_message }}
{{filter}}


