METRONOMIC CHEMOTHERAPY IMPROVES SURVIVAL IN RESPONDING PATIENTS WITH RECURRENT/REFRACTORY LYMPHOMA
(Abstract release date: 05/19/16)
EHA Library. Sanchez-Gonzalez B. 06/09/16; 134625; PB1725

Dr. Blanca Sanchez-Gonzalez
Contributions
Contributions
Abstract
Abstract: PB1725
Type: Publication Only
Background
Metronomic chemotherapy (MC) consists of continuous administration of oral chemotherapy at low, potentially less toxic doses without prolonged drug-free breaks. MC might be a useful for many patients with recurrent or refractory lymphomas that are unable to tolerate intensive therapies
Aims
The aim of this study was to retrospectively analyze the efficacy and toxicity of MC in recurrent or refractory lymphomas in our Institution
Methods
Retrospective analysis of patients with lymphoma treated with MC from 2009 to2014 in our Institution.The metronomic scheme consisted of oral 50 mg of prednisone, 50 mg of cyclophosphamide, 50 mg of etoposide, and/or 50 mg of procarbazine evenly distributed throughout the day.Clinical response, duration of response, progression-free survival (PFS) and overall survival (OS) was evaluated. Clinical response was defined as improvement of the symptoms of lymphoma and / or shrinkage of tumours (lymph nodes or affected organs) by physical examination and / or imaging
Results
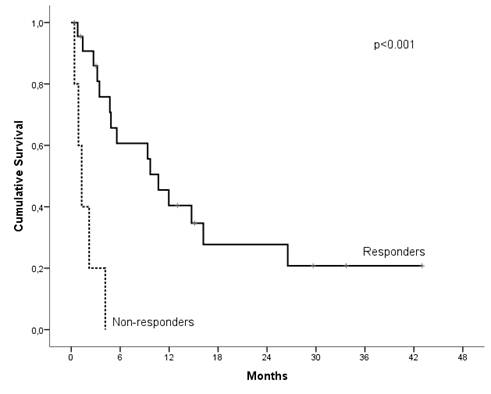
Patient demographics and characteristics: 28 lymphoma patients consecutively treated with MC were included. 10 patients had diffuse large B-cell lymphoma (DLBCL), 6 cutaneous T-cell lymphoma (CTCL), 5 peripheral T-cell lymphoma (T-NHL), 4 Hodgkin lymphoma (HL), 1 mantle cell lymphoma, 2 other types of non-Hodgkin lymphoma. Median number of prior regimens was 3 (range 1-8). 26 patients (93%) had refractory disease to prior treatment. Efficacy: Clinical response was observed in 23 patients (82%) with a median duration of response 6 months (95% CI, 0-11 months). No differences were found in clinical response rate, duration of response, PFS and OS among the aggressive or indolent lymphomas. With a median follow-up of 14 months, median OS was 6 months. Of note, responders to MC showed a significantly increased OS (median OS of 11 months in responders vs 1 months in non-responders (p<0.001)(figure 1). Remarkably, PFS was also significantly higher in responders, 42% of cases were progression-free at 6 months in responders vs 0% in non-responders (p<0.001). Twenty patients died: progressive disease (n=15), infection (n=4) and non-related (n=1).Toxicity: Seventeen patients (61%) had adverse events grade 3/4, mainly hematologic. A total of 6 patients had infections grade ≥ 3: urinary tract infection (n=2), pneumonia (n=2), multiresistant Pseudomonas aeruginosa bacteremia + Cytomegalovirus infection (n=1) and Escherichia coli sepsis (n=1). The main cause of treatment discontinuation was progressive disease. Only one gastrointestinal adverse event grade 4 led to MC discontinuation (3.6%). Dose modifications of MC drugs was performed in 15 patients (54%) and 12 patients required use of granulocyte colony stimulating factor (G-CSF). 54% of patients received cotrimoxazole as primary or secondary prophylaxis
Conclusion
Our study supports the anti-tumor activity of MC in patients with advanced lymphoma chemo-resistant and relapsed to multiple therapies with no other therapeutic alternatives. Clinical responses were observed in all lymphoma subgroups and the toxicity profile was acceptable, even in heavily pretreated patients. Even most of our cases had been considered refractory to prior treatment, responding patients to MC had an improved PFS and even OS
Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, Non-Hodgkin's lymphoma, Treatment, Treatment-related mortality
Type: Publication Only
Background
Metronomic chemotherapy (MC) consists of continuous administration of oral chemotherapy at low, potentially less toxic doses without prolonged drug-free breaks. MC might be a useful for many patients with recurrent or refractory lymphomas that are unable to tolerate intensive therapies
Aims
The aim of this study was to retrospectively analyze the efficacy and toxicity of MC in recurrent or refractory lymphomas in our Institution
Methods
Retrospective analysis of patients with lymphoma treated with MC from 2009 to2014 in our Institution.The metronomic scheme consisted of oral 50 mg of prednisone, 50 mg of cyclophosphamide, 50 mg of etoposide, and/or 50 mg of procarbazine evenly distributed throughout the day.Clinical response, duration of response, progression-free survival (PFS) and overall survival (OS) was evaluated. Clinical response was defined as improvement of the symptoms of lymphoma and / or shrinkage of tumours (lymph nodes or affected organs) by physical examination and / or imaging
Results
Patient demographics and characteristics: 28 lymphoma patients consecutively treated with MC were included. 10 patients had diffuse large B-cell lymphoma (DLBCL), 6 cutaneous T-cell lymphoma (CTCL), 5 peripheral T-cell lymphoma (T-NHL), 4 Hodgkin lymphoma (HL), 1 mantle cell lymphoma, 2 other types of non-Hodgkin lymphoma. Median number of prior regimens was 3 (range 1-8). 26 patients (93%) had refractory disease to prior treatment. Efficacy: Clinical response was observed in 23 patients (82%) with a median duration of response 6 months (95% CI, 0-11 months). No differences were found in clinical response rate, duration of response, PFS and OS among the aggressive or indolent lymphomas. With a median follow-up of 14 months, median OS was 6 months. Of note, responders to MC showed a significantly increased OS (median OS of 11 months in responders vs 1 months in non-responders (p<0.001)(figure 1). Remarkably, PFS was also significantly higher in responders, 42% of cases were progression-free at 6 months in responders vs 0% in non-responders (p<0.001). Twenty patients died: progressive disease (n=15), infection (n=4) and non-related (n=1).Toxicity: Seventeen patients (61%) had adverse events grade 3/4, mainly hematologic. A total of 6 patients had infections grade ≥ 3: urinary tract infection (n=2), pneumonia (n=2), multiresistant Pseudomonas aeruginosa bacteremia + Cytomegalovirus infection (n=1) and Escherichia coli sepsis (n=1). The main cause of treatment discontinuation was progressive disease. Only one gastrointestinal adverse event grade 4 led to MC discontinuation (3.6%). Dose modifications of MC drugs was performed in 15 patients (54%) and 12 patients required use of granulocyte colony stimulating factor (G-CSF). 54% of patients received cotrimoxazole as primary or secondary prophylaxis
Conclusion
Our study supports the anti-tumor activity of MC in patients with advanced lymphoma chemo-resistant and relapsed to multiple therapies with no other therapeutic alternatives. Clinical responses were observed in all lymphoma subgroups and the toxicity profile was acceptable, even in heavily pretreated patients. Even most of our cases had been considered refractory to prior treatment, responding patients to MC had an improved PFS and even OS

Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, Non-Hodgkin's lymphoma, Treatment, Treatment-related mortality
Abstract: PB1725
Type: Publication Only
Background
Metronomic chemotherapy (MC) consists of continuous administration of oral chemotherapy at low, potentially less toxic doses without prolonged drug-free breaks. MC might be a useful for many patients with recurrent or refractory lymphomas that are unable to tolerate intensive therapies
Aims
The aim of this study was to retrospectively analyze the efficacy and toxicity of MC in recurrent or refractory lymphomas in our Institution
Methods
Retrospective analysis of patients with lymphoma treated with MC from 2009 to2014 in our Institution.The metronomic scheme consisted of oral 50 mg of prednisone, 50 mg of cyclophosphamide, 50 mg of etoposide, and/or 50 mg of procarbazine evenly distributed throughout the day.Clinical response, duration of response, progression-free survival (PFS) and overall survival (OS) was evaluated. Clinical response was defined as improvement of the symptoms of lymphoma and / or shrinkage of tumours (lymph nodes or affected organs) by physical examination and / or imaging
Results
Patient demographics and characteristics: 28 lymphoma patients consecutively treated with MC were included. 10 patients had diffuse large B-cell lymphoma (DLBCL), 6 cutaneous T-cell lymphoma (CTCL), 5 peripheral T-cell lymphoma (T-NHL), 4 Hodgkin lymphoma (HL), 1 mantle cell lymphoma, 2 other types of non-Hodgkin lymphoma. Median number of prior regimens was 3 (range 1-8). 26 patients (93%) had refractory disease to prior treatment. Efficacy: Clinical response was observed in 23 patients (82%) with a median duration of response 6 months (95% CI, 0-11 months). No differences were found in clinical response rate, duration of response, PFS and OS among the aggressive or indolent lymphomas. With a median follow-up of 14 months, median OS was 6 months. Of note, responders to MC showed a significantly increased OS (median OS of 11 months in responders vs 1 months in non-responders (p<0.001)(figure 1). Remarkably, PFS was also significantly higher in responders, 42% of cases were progression-free at 6 months in responders vs 0% in non-responders (p<0.001). Twenty patients died: progressive disease (n=15), infection (n=4) and non-related (n=1).Toxicity: Seventeen patients (61%) had adverse events grade 3/4, mainly hematologic. A total of 6 patients had infections grade ≥ 3: urinary tract infection (n=2), pneumonia (n=2), multiresistant Pseudomonas aeruginosa bacteremia + Cytomegalovirus infection (n=1) and Escherichia coli sepsis (n=1). The main cause of treatment discontinuation was progressive disease. Only one gastrointestinal adverse event grade 4 led to MC discontinuation (3.6%). Dose modifications of MC drugs was performed in 15 patients (54%) and 12 patients required use of granulocyte colony stimulating factor (G-CSF). 54% of patients received cotrimoxazole as primary or secondary prophylaxis
Conclusion
Our study supports the anti-tumor activity of MC in patients with advanced lymphoma chemo-resistant and relapsed to multiple therapies with no other therapeutic alternatives. Clinical responses were observed in all lymphoma subgroups and the toxicity profile was acceptable, even in heavily pretreated patients. Even most of our cases had been considered refractory to prior treatment, responding patients to MC had an improved PFS and even OS

Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, Non-Hodgkin's lymphoma, Treatment, Treatment-related mortality
Type: Publication Only
Background
Metronomic chemotherapy (MC) consists of continuous administration of oral chemotherapy at low, potentially less toxic doses without prolonged drug-free breaks. MC might be a useful for many patients with recurrent or refractory lymphomas that are unable to tolerate intensive therapies
Aims
The aim of this study was to retrospectively analyze the efficacy and toxicity of MC in recurrent or refractory lymphomas in our Institution
Methods
Retrospective analysis of patients with lymphoma treated with MC from 2009 to2014 in our Institution.The metronomic scheme consisted of oral 50 mg of prednisone, 50 mg of cyclophosphamide, 50 mg of etoposide, and/or 50 mg of procarbazine evenly distributed throughout the day.Clinical response, duration of response, progression-free survival (PFS) and overall survival (OS) was evaluated. Clinical response was defined as improvement of the symptoms of lymphoma and / or shrinkage of tumours (lymph nodes or affected organs) by physical examination and / or imaging
Results
Patient demographics and characteristics: 28 lymphoma patients consecutively treated with MC were included. 10 patients had diffuse large B-cell lymphoma (DLBCL), 6 cutaneous T-cell lymphoma (CTCL), 5 peripheral T-cell lymphoma (T-NHL), 4 Hodgkin lymphoma (HL), 1 mantle cell lymphoma, 2 other types of non-Hodgkin lymphoma. Median number of prior regimens was 3 (range 1-8). 26 patients (93%) had refractory disease to prior treatment. Efficacy: Clinical response was observed in 23 patients (82%) with a median duration of response 6 months (95% CI, 0-11 months). No differences were found in clinical response rate, duration of response, PFS and OS among the aggressive or indolent lymphomas. With a median follow-up of 14 months, median OS was 6 months. Of note, responders to MC showed a significantly increased OS (median OS of 11 months in responders vs 1 months in non-responders (p<0.001)(figure 1). Remarkably, PFS was also significantly higher in responders, 42% of cases were progression-free at 6 months in responders vs 0% in non-responders (p<0.001). Twenty patients died: progressive disease (n=15), infection (n=4) and non-related (n=1).Toxicity: Seventeen patients (61%) had adverse events grade 3/4, mainly hematologic. A total of 6 patients had infections grade ≥ 3: urinary tract infection (n=2), pneumonia (n=2), multiresistant Pseudomonas aeruginosa bacteremia + Cytomegalovirus infection (n=1) and Escherichia coli sepsis (n=1). The main cause of treatment discontinuation was progressive disease. Only one gastrointestinal adverse event grade 4 led to MC discontinuation (3.6%). Dose modifications of MC drugs was performed in 15 patients (54%) and 12 patients required use of granulocyte colony stimulating factor (G-CSF). 54% of patients received cotrimoxazole as primary or secondary prophylaxis
Conclusion
Our study supports the anti-tumor activity of MC in patients with advanced lymphoma chemo-resistant and relapsed to multiple therapies with no other therapeutic alternatives. Clinical responses were observed in all lymphoma subgroups and the toxicity profile was acceptable, even in heavily pretreated patients. Even most of our cases had been considered refractory to prior treatment, responding patients to MC had an improved PFS and even OS

Session topic: E-poster
Keyword(s): Hodgkin's lymphoma, Non-Hodgkin's lymphoma, Treatment, Treatment-related mortality
{{ help_message }}
{{filter}}


