EFFICACY AND SAFETY OF ADJUSTED CAG IN COMBINATION WITH DECITABINE IN NEWLY DIAGNOSED OR REFRACTORY/RELAPSED ELDERLY PATIENTS WITH ACUTE MYELOID LEUKEMIA
(Abstract release date: 05/19/16)
EHA Library. Jiang Q. 06/09/16; 134559; PB1659

Qianli Jiang
Contributions
Contributions
Abstract
Abstract: PB1659
Type: Publication Only
Background
Priming regimen(e.g., CAG) combined with Decitabine is increasingly being used to treat acute myeloid leukemia, especially in elderly patients. However, the particular mode of administration and dosage of Decitabine has not been unified.
Aims
This pilot study was designed to evaluate the efficacy and safety of adjusted CAG (aCAG) regimen combined with Decitabine treatment for elderly patients with newly diagnosed or refractory/relapsed acute myeloid leukemia (AML).
Methods
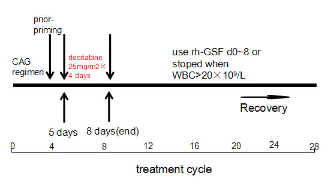
All patients (age≥60 years, PS≤2) in this study were treated with aCAG-D regimen: Cytarabine (10mg/m2/12h, days 1-8), Aclarubicin (5-7mg/m2/day, days 1-8), Granulocyte colony-stimulating factor (300μg/day, days 0-8 or stopped when WBC count >20×109/L) and Decitabine(25mg/m2/day, days 5-8) for two cycles.
Results
The median age of the twelve patients (seven men and five women) able to be evaluated, which contained nine de novo patients and three refractory/relapsed patients, was 67 years (range of 60-75 years). A total of nine patients (75.0%) achieved complete remission (CR) after the aCAG-D treatment, including eight of whom achieved CR after only one treatment cycle. In nine newly diagnosed patients eight cases achieved CR (88.9%) and another got partial remission (PR). Meanwhile, three other refractory/relapsed patients achieved CR/ partial remission (PR)/no response (NR) respectively. The overall response rate (ORR) and CR after two treatment cycles were 91.6% and 75.0%, respectively. In patients with CR, the median time was 24.2 days (range of 16-43 days) for granulocyte recovery and 17.9 days (range of 11-37 days) for platelet recovery. After a median follow-up of 11 months (range of 4.5-22 months), one patient died due to secondary infections; one patient showed a relapse; five patients died due to progression of the disease; five patients maintained CR. The median overall survival (OS) of 13 months(range of 4.5-22 months) for those who achieved CR was significantly longer than that of patients who did not achieved CR(5 months). The main adverse events were grade Ⅳ bone marrow suppression(12/12 patients) and grade Ⅰ-Ⅳ secondary infection(11/12 patients). There was no treatment-related mortality during remission induction.
Conclusion
The regimen combined aCAG and Decitabine, which designed to use CAG scheme for priming firstly, then add Decitabine to target tumor cells in the S-phase of the cell cycle, demonstrates good efficacy for elderly AML patients, especially in de novo patients. Meanwhile, this regimen didn’t give rise to serious myelo-suppression and secondary infection. In general, aCAG-D regimen was well tolerated by the elderly patients with de novo or relapsed/refractory AML and showed promising clinical efficacy.
Session topic: E-poster
Keyword(s): AML, Decitabine, Elderly
Type: Publication Only
Background
Priming regimen(e.g., CAG) combined with Decitabine is increasingly being used to treat acute myeloid leukemia, especially in elderly patients. However, the particular mode of administration and dosage of Decitabine has not been unified.
Aims
This pilot study was designed to evaluate the efficacy and safety of adjusted CAG (aCAG) regimen combined with Decitabine treatment for elderly patients with newly diagnosed or refractory/relapsed acute myeloid leukemia (AML).
Methods
All patients (age≥60 years, PS≤2) in this study were treated with aCAG-D regimen: Cytarabine (10mg/m2/12h, days 1-8), Aclarubicin (5-7mg/m2/day, days 1-8), Granulocyte colony-stimulating factor (300μg/day, days 0-8 or stopped when WBC count >20×109/L) and Decitabine(25mg/m2/day, days 5-8) for two cycles.
Results
The median age of the twelve patients (seven men and five women) able to be evaluated, which contained nine de novo patients and three refractory/relapsed patients, was 67 years (range of 60-75 years). A total of nine patients (75.0%) achieved complete remission (CR) after the aCAG-D treatment, including eight of whom achieved CR after only one treatment cycle. In nine newly diagnosed patients eight cases achieved CR (88.9%) and another got partial remission (PR). Meanwhile, three other refractory/relapsed patients achieved CR/ partial remission (PR)/no response (NR) respectively. The overall response rate (ORR) and CR after two treatment cycles were 91.6% and 75.0%, respectively. In patients with CR, the median time was 24.2 days (range of 16-43 days) for granulocyte recovery and 17.9 days (range of 11-37 days) for platelet recovery. After a median follow-up of 11 months (range of 4.5-22 months), one patient died due to secondary infections; one patient showed a relapse; five patients died due to progression of the disease; five patients maintained CR. The median overall survival (OS) of 13 months(range of 4.5-22 months) for those who achieved CR was significantly longer than that of patients who did not achieved CR(5 months). The main adverse events were grade Ⅳ bone marrow suppression(12/12 patients) and grade Ⅰ-Ⅳ secondary infection(11/12 patients). There was no treatment-related mortality during remission induction.
Conclusion
The regimen combined aCAG and Decitabine, which designed to use CAG scheme for priming firstly, then add Decitabine to target tumor cells in the S-phase of the cell cycle, demonstrates good efficacy for elderly AML patients, especially in de novo patients. Meanwhile, this regimen didn’t give rise to serious myelo-suppression and secondary infection. In general, aCAG-D regimen was well tolerated by the elderly patients with de novo or relapsed/refractory AML and showed promising clinical efficacy.

Session topic: E-poster
Keyword(s): AML, Decitabine, Elderly
Abstract: PB1659
Type: Publication Only
Background
Priming regimen(e.g., CAG) combined with Decitabine is increasingly being used to treat acute myeloid leukemia, especially in elderly patients. However, the particular mode of administration and dosage of Decitabine has not been unified.
Aims
This pilot study was designed to evaluate the efficacy and safety of adjusted CAG (aCAG) regimen combined with Decitabine treatment for elderly patients with newly diagnosed or refractory/relapsed acute myeloid leukemia (AML).
Methods
All patients (age≥60 years, PS≤2) in this study were treated with aCAG-D regimen: Cytarabine (10mg/m2/12h, days 1-8), Aclarubicin (5-7mg/m2/day, days 1-8), Granulocyte colony-stimulating factor (300μg/day, days 0-8 or stopped when WBC count >20×109/L) and Decitabine(25mg/m2/day, days 5-8) for two cycles.
Results
The median age of the twelve patients (seven men and five women) able to be evaluated, which contained nine de novo patients and three refractory/relapsed patients, was 67 years (range of 60-75 years). A total of nine patients (75.0%) achieved complete remission (CR) after the aCAG-D treatment, including eight of whom achieved CR after only one treatment cycle. In nine newly diagnosed patients eight cases achieved CR (88.9%) and another got partial remission (PR). Meanwhile, three other refractory/relapsed patients achieved CR/ partial remission (PR)/no response (NR) respectively. The overall response rate (ORR) and CR after two treatment cycles were 91.6% and 75.0%, respectively. In patients with CR, the median time was 24.2 days (range of 16-43 days) for granulocyte recovery and 17.9 days (range of 11-37 days) for platelet recovery. After a median follow-up of 11 months (range of 4.5-22 months), one patient died due to secondary infections; one patient showed a relapse; five patients died due to progression of the disease; five patients maintained CR. The median overall survival (OS) of 13 months(range of 4.5-22 months) for those who achieved CR was significantly longer than that of patients who did not achieved CR(5 months). The main adverse events were grade Ⅳ bone marrow suppression(12/12 patients) and grade Ⅰ-Ⅳ secondary infection(11/12 patients). There was no treatment-related mortality during remission induction.
Conclusion
The regimen combined aCAG and Decitabine, which designed to use CAG scheme for priming firstly, then add Decitabine to target tumor cells in the S-phase of the cell cycle, demonstrates good efficacy for elderly AML patients, especially in de novo patients. Meanwhile, this regimen didn’t give rise to serious myelo-suppression and secondary infection. In general, aCAG-D regimen was well tolerated by the elderly patients with de novo or relapsed/refractory AML and showed promising clinical efficacy.

Session topic: E-poster
Keyword(s): AML, Decitabine, Elderly
Type: Publication Only
Background
Priming regimen(e.g., CAG) combined with Decitabine is increasingly being used to treat acute myeloid leukemia, especially in elderly patients. However, the particular mode of administration and dosage of Decitabine has not been unified.
Aims
This pilot study was designed to evaluate the efficacy and safety of adjusted CAG (aCAG) regimen combined with Decitabine treatment for elderly patients with newly diagnosed or refractory/relapsed acute myeloid leukemia (AML).
Methods
All patients (age≥60 years, PS≤2) in this study were treated with aCAG-D regimen: Cytarabine (10mg/m2/12h, days 1-8), Aclarubicin (5-7mg/m2/day, days 1-8), Granulocyte colony-stimulating factor (300μg/day, days 0-8 or stopped when WBC count >20×109/L) and Decitabine(25mg/m2/day, days 5-8) for two cycles.
Results
The median age of the twelve patients (seven men and five women) able to be evaluated, which contained nine de novo patients and three refractory/relapsed patients, was 67 years (range of 60-75 years). A total of nine patients (75.0%) achieved complete remission (CR) after the aCAG-D treatment, including eight of whom achieved CR after only one treatment cycle. In nine newly diagnosed patients eight cases achieved CR (88.9%) and another got partial remission (PR). Meanwhile, three other refractory/relapsed patients achieved CR/ partial remission (PR)/no response (NR) respectively. The overall response rate (ORR) and CR after two treatment cycles were 91.6% and 75.0%, respectively. In patients with CR, the median time was 24.2 days (range of 16-43 days) for granulocyte recovery and 17.9 days (range of 11-37 days) for platelet recovery. After a median follow-up of 11 months (range of 4.5-22 months), one patient died due to secondary infections; one patient showed a relapse; five patients died due to progression of the disease; five patients maintained CR. The median overall survival (OS) of 13 months(range of 4.5-22 months) for those who achieved CR was significantly longer than that of patients who did not achieved CR(5 months). The main adverse events were grade Ⅳ bone marrow suppression(12/12 patients) and grade Ⅰ-Ⅳ secondary infection(11/12 patients). There was no treatment-related mortality during remission induction.
Conclusion
The regimen combined aCAG and Decitabine, which designed to use CAG scheme for priming firstly, then add Decitabine to target tumor cells in the S-phase of the cell cycle, demonstrates good efficacy for elderly AML patients, especially in de novo patients. Meanwhile, this regimen didn’t give rise to serious myelo-suppression and secondary infection. In general, aCAG-D regimen was well tolerated by the elderly patients with de novo or relapsed/refractory AML and showed promising clinical efficacy.

Session topic: E-poster
Keyword(s): AML, Decitabine, Elderly
{{ help_message }}
{{filter}}


