TRANSPLACENTAL CARCINOGEN EXPOSURE INCREASES THE LEVELS OF DNA DAMAGEA IN HUMAN UMBILICAL CORD BLOOD AND MOUSE C57BL/6 BONE MARROW CELLS
(Abstract release date: 05/19/16)
EHA Library. Zareian N. 06/09/16; 134490; PB1590

Ms. Nahid Zareian
Contributions
Contributions
Abstract
Abstract: PB1590
Type: Publication Only
Background
Acute leukaemia is the principal subtype of paediatric cancer and, despite success in treatment, its aetiology remains unclear. Maternal exposure to radiation and benzene metabolites during foetal development has been implicated in its aetiology as the first hit in the context of a minimal two-hit model of the natural history of the disease. Although there might not be an exclusive cause, an abnormal immune response to childhood infections has been proposed as the plausible aetiology. We have previously shown that a human placental barrier responds to different types of toxic challenge and oxidative stress by secreting molecules that cause DNA damage and chromosome aberrations in different cell types distal to the barrier including human fibroblast and human embryonic stem cells.
Aims
We aimed to investigate: a. the aetiology of childhood leukaemia by focusing on the role of the placenta in foetal leukaemogenesis; b. the effect of nanoparticles (NP) as drug delivery molecules on preventing DNA lesions during pregnancy.
Methods
In vitro. Human umbilical cord blood (UCB) cells were exposed indirectly to 30 µM hydroquinone (HQ) and benzoquinone (BQ) across bilayered cell barriers of human trophoblast choriocarcinoma-derived cell line BeWo grown on transwell inserts (pore size, 0.4 mm). Media with no HQ and BQ was the control. Ex vivo. To investigate the effect of radiation-induced bystander mechanism and pharmacological treatment on preventing DNA damages, female C57Bl/6J mice at day 12 gestation were exposed to a whole body exposure non-irradiated, 100 mGy or 1 Gy X-irradiation (AGO, United Kingdom, dose rate 0.5 Gy/min (250 kVp and 13 mA)); the placentae were removed 4 hrs post-irradiation and cultured with 3 conditions: with no drug additive, with MitoQ bound to NP (MQNP) and with blank NP (BNP) as the control for MQNP. Total bone marrow (BM) from age-matched female C57Bl/6J was exposed to conditioned media. DNA damage was measured using quantitative immunocytochemistry for a panel of DNA damage markers including γH2AX and 53BP1 (DNA double-strand break (DSB)) and FANCD2 (interstrand crosslinks (ICL)). The experiments were carried out in triplicate.
Results
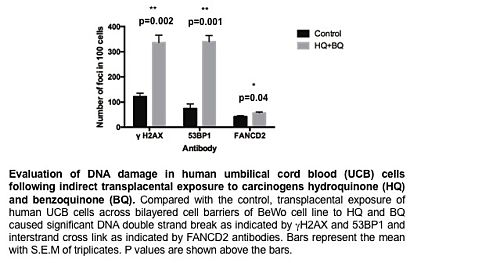
Exposure of UCB to HQ and BQ across BeWo barrier increased level of DNA damage. UCB cells showed significantly more damage after 24 hrs exposure (γH2AX (p=0.002), 53BP1 (p=0.001) and FANCD2 (p=0.04), Figure 1).Exposure of murine BM cells to conditioned media with no drug additive increased DNA DSB and ICL. BM γH2AX-positive cells showed increased DNA DSB at 1 Gy (p=0.006) but the difference was not statistically significant at 100 mGy (p=0.07). BM 53BP1-positive and BM FANCD2-positive cells showed significantly more DNA damage compared to their control counterparts at both 100 mGy and 1 Gy doses (p=0.01 and p=0.001, p=0.02 and p=0.0001, respectively). MQNP prevented DNA damaging secretions in BM treated cells however this effect was more pronounced in BM 53BP1-positive and BM FANCD2-positive cells at both 100 mGy and 1 Gy doses (γH2AX (p=0.1 and p=0.06), 53BP1 (p=0.002 and p=0.02) and FANCD2 (p=0.02 and p=0.1), respectively).
Conclusion
Our data show that transplacental exposure of human UCB and murine BM cells to carcinogens BQ/HQ and irradiation increases levels of DNA damage presumably leading to increased genome instability. Furthermore, a marked increased phosphorylation of histone H2AX, 53BP1 and FANCD2 in BM cells indicates the effect of a radiation-induced bystander mechanism. DNA damaging secretion conferred by irradiation exposure in the ex vivo model could be prevented by applying nanotechnology-based drug delivery molecules to the placental conditioned medium and further investigations are ongoing. These findings suggest the importance of placenta as a barrier in DNA damage signal propagation during embryo development and provide new insights into the link between placental signaling and foetal leukaemogenesis. Whether these events lead to the accumulation of further genetic aberrations in the context of a minimal two-hit model for leukaemia initiation remains to be further elucidated.
Session topic: E-poster
Keyword(s): Acute leukemia
Type: Publication Only
Background
Acute leukaemia is the principal subtype of paediatric cancer and, despite success in treatment, its aetiology remains unclear. Maternal exposure to radiation and benzene metabolites during foetal development has been implicated in its aetiology as the first hit in the context of a minimal two-hit model of the natural history of the disease. Although there might not be an exclusive cause, an abnormal immune response to childhood infections has been proposed as the plausible aetiology. We have previously shown that a human placental barrier responds to different types of toxic challenge and oxidative stress by secreting molecules that cause DNA damage and chromosome aberrations in different cell types distal to the barrier including human fibroblast and human embryonic stem cells.
Aims
We aimed to investigate: a. the aetiology of childhood leukaemia by focusing on the role of the placenta in foetal leukaemogenesis; b. the effect of nanoparticles (NP) as drug delivery molecules on preventing DNA lesions during pregnancy.
Methods
In vitro. Human umbilical cord blood (UCB) cells were exposed indirectly to 30 µM hydroquinone (HQ) and benzoquinone (BQ) across bilayered cell barriers of human trophoblast choriocarcinoma-derived cell line BeWo grown on transwell inserts (pore size, 0.4 mm). Media with no HQ and BQ was the control. Ex vivo. To investigate the effect of radiation-induced bystander mechanism and pharmacological treatment on preventing DNA damages, female C57Bl/6J mice at day 12 gestation were exposed to a whole body exposure non-irradiated, 100 mGy or 1 Gy X-irradiation (AGO, United Kingdom, dose rate 0.5 Gy/min (250 kVp and 13 mA)); the placentae were removed 4 hrs post-irradiation and cultured with 3 conditions: with no drug additive, with MitoQ bound to NP (MQNP) and with blank NP (BNP) as the control for MQNP. Total bone marrow (BM) from age-matched female C57Bl/6J was exposed to conditioned media. DNA damage was measured using quantitative immunocytochemistry for a panel of DNA damage markers including γH2AX and 53BP1 (DNA double-strand break (DSB)) and FANCD2 (interstrand crosslinks (ICL)). The experiments were carried out in triplicate.
Results
Exposure of UCB to HQ and BQ across BeWo barrier increased level of DNA damage. UCB cells showed significantly more damage after 24 hrs exposure (γH2AX (p=0.002), 53BP1 (p=0.001) and FANCD2 (p=0.04), Figure 1).Exposure of murine BM cells to conditioned media with no drug additive increased DNA DSB and ICL. BM γH2AX-positive cells showed increased DNA DSB at 1 Gy (p=0.006) but the difference was not statistically significant at 100 mGy (p=0.07). BM 53BP1-positive and BM FANCD2-positive cells showed significantly more DNA damage compared to their control counterparts at both 100 mGy and 1 Gy doses (p=0.01 and p=0.001, p=0.02 and p=0.0001, respectively). MQNP prevented DNA damaging secretions in BM treated cells however this effect was more pronounced in BM 53BP1-positive and BM FANCD2-positive cells at both 100 mGy and 1 Gy doses (γH2AX (p=0.1 and p=0.06), 53BP1 (p=0.002 and p=0.02) and FANCD2 (p=0.02 and p=0.1), respectively).
Conclusion
Our data show that transplacental exposure of human UCB and murine BM cells to carcinogens BQ/HQ and irradiation increases levels of DNA damage presumably leading to increased genome instability. Furthermore, a marked increased phosphorylation of histone H2AX, 53BP1 and FANCD2 in BM cells indicates the effect of a radiation-induced bystander mechanism. DNA damaging secretion conferred by irradiation exposure in the ex vivo model could be prevented by applying nanotechnology-based drug delivery molecules to the placental conditioned medium and further investigations are ongoing. These findings suggest the importance of placenta as a barrier in DNA damage signal propagation during embryo development and provide new insights into the link between placental signaling and foetal leukaemogenesis. Whether these events lead to the accumulation of further genetic aberrations in the context of a minimal two-hit model for leukaemia initiation remains to be further elucidated.

Session topic: E-poster
Keyword(s): Acute leukemia
Abstract: PB1590
Type: Publication Only
Background
Acute leukaemia is the principal subtype of paediatric cancer and, despite success in treatment, its aetiology remains unclear. Maternal exposure to radiation and benzene metabolites during foetal development has been implicated in its aetiology as the first hit in the context of a minimal two-hit model of the natural history of the disease. Although there might not be an exclusive cause, an abnormal immune response to childhood infections has been proposed as the plausible aetiology. We have previously shown that a human placental barrier responds to different types of toxic challenge and oxidative stress by secreting molecules that cause DNA damage and chromosome aberrations in different cell types distal to the barrier including human fibroblast and human embryonic stem cells.
Aims
We aimed to investigate: a. the aetiology of childhood leukaemia by focusing on the role of the placenta in foetal leukaemogenesis; b. the effect of nanoparticles (NP) as drug delivery molecules on preventing DNA lesions during pregnancy.
Methods
In vitro. Human umbilical cord blood (UCB) cells were exposed indirectly to 30 µM hydroquinone (HQ) and benzoquinone (BQ) across bilayered cell barriers of human trophoblast choriocarcinoma-derived cell line BeWo grown on transwell inserts (pore size, 0.4 mm). Media with no HQ and BQ was the control. Ex vivo. To investigate the effect of radiation-induced bystander mechanism and pharmacological treatment on preventing DNA damages, female C57Bl/6J mice at day 12 gestation were exposed to a whole body exposure non-irradiated, 100 mGy or 1 Gy X-irradiation (AGO, United Kingdom, dose rate 0.5 Gy/min (250 kVp and 13 mA)); the placentae were removed 4 hrs post-irradiation and cultured with 3 conditions: with no drug additive, with MitoQ bound to NP (MQNP) and with blank NP (BNP) as the control for MQNP. Total bone marrow (BM) from age-matched female C57Bl/6J was exposed to conditioned media. DNA damage was measured using quantitative immunocytochemistry for a panel of DNA damage markers including γH2AX and 53BP1 (DNA double-strand break (DSB)) and FANCD2 (interstrand crosslinks (ICL)). The experiments were carried out in triplicate.
Results
Exposure of UCB to HQ and BQ across BeWo barrier increased level of DNA damage. UCB cells showed significantly more damage after 24 hrs exposure (γH2AX (p=0.002), 53BP1 (p=0.001) and FANCD2 (p=0.04), Figure 1).Exposure of murine BM cells to conditioned media with no drug additive increased DNA DSB and ICL. BM γH2AX-positive cells showed increased DNA DSB at 1 Gy (p=0.006) but the difference was not statistically significant at 100 mGy (p=0.07). BM 53BP1-positive and BM FANCD2-positive cells showed significantly more DNA damage compared to their control counterparts at both 100 mGy and 1 Gy doses (p=0.01 and p=0.001, p=0.02 and p=0.0001, respectively). MQNP prevented DNA damaging secretions in BM treated cells however this effect was more pronounced in BM 53BP1-positive and BM FANCD2-positive cells at both 100 mGy and 1 Gy doses (γH2AX (p=0.1 and p=0.06), 53BP1 (p=0.002 and p=0.02) and FANCD2 (p=0.02 and p=0.1), respectively).
Conclusion
Our data show that transplacental exposure of human UCB and murine BM cells to carcinogens BQ/HQ and irradiation increases levels of DNA damage presumably leading to increased genome instability. Furthermore, a marked increased phosphorylation of histone H2AX, 53BP1 and FANCD2 in BM cells indicates the effect of a radiation-induced bystander mechanism. DNA damaging secretion conferred by irradiation exposure in the ex vivo model could be prevented by applying nanotechnology-based drug delivery molecules to the placental conditioned medium and further investigations are ongoing. These findings suggest the importance of placenta as a barrier in DNA damage signal propagation during embryo development and provide new insights into the link between placental signaling and foetal leukaemogenesis. Whether these events lead to the accumulation of further genetic aberrations in the context of a minimal two-hit model for leukaemia initiation remains to be further elucidated.

Session topic: E-poster
Keyword(s): Acute leukemia
Type: Publication Only
Background
Acute leukaemia is the principal subtype of paediatric cancer and, despite success in treatment, its aetiology remains unclear. Maternal exposure to radiation and benzene metabolites during foetal development has been implicated in its aetiology as the first hit in the context of a minimal two-hit model of the natural history of the disease. Although there might not be an exclusive cause, an abnormal immune response to childhood infections has been proposed as the plausible aetiology. We have previously shown that a human placental barrier responds to different types of toxic challenge and oxidative stress by secreting molecules that cause DNA damage and chromosome aberrations in different cell types distal to the barrier including human fibroblast and human embryonic stem cells.
Aims
We aimed to investigate: a. the aetiology of childhood leukaemia by focusing on the role of the placenta in foetal leukaemogenesis; b. the effect of nanoparticles (NP) as drug delivery molecules on preventing DNA lesions during pregnancy.
Methods
In vitro. Human umbilical cord blood (UCB) cells were exposed indirectly to 30 µM hydroquinone (HQ) and benzoquinone (BQ) across bilayered cell barriers of human trophoblast choriocarcinoma-derived cell line BeWo grown on transwell inserts (pore size, 0.4 mm). Media with no HQ and BQ was the control. Ex vivo. To investigate the effect of radiation-induced bystander mechanism and pharmacological treatment on preventing DNA damages, female C57Bl/6J mice at day 12 gestation were exposed to a whole body exposure non-irradiated, 100 mGy or 1 Gy X-irradiation (AGO, United Kingdom, dose rate 0.5 Gy/min (250 kVp and 13 mA)); the placentae were removed 4 hrs post-irradiation and cultured with 3 conditions: with no drug additive, with MitoQ bound to NP (MQNP) and with blank NP (BNP) as the control for MQNP. Total bone marrow (BM) from age-matched female C57Bl/6J was exposed to conditioned media. DNA damage was measured using quantitative immunocytochemistry for a panel of DNA damage markers including γH2AX and 53BP1 (DNA double-strand break (DSB)) and FANCD2 (interstrand crosslinks (ICL)). The experiments were carried out in triplicate.
Results
Exposure of UCB to HQ and BQ across BeWo barrier increased level of DNA damage. UCB cells showed significantly more damage after 24 hrs exposure (γH2AX (p=0.002), 53BP1 (p=0.001) and FANCD2 (p=0.04), Figure 1).Exposure of murine BM cells to conditioned media with no drug additive increased DNA DSB and ICL. BM γH2AX-positive cells showed increased DNA DSB at 1 Gy (p=0.006) but the difference was not statistically significant at 100 mGy (p=0.07). BM 53BP1-positive and BM FANCD2-positive cells showed significantly more DNA damage compared to their control counterparts at both 100 mGy and 1 Gy doses (p=0.01 and p=0.001, p=0.02 and p=0.0001, respectively). MQNP prevented DNA damaging secretions in BM treated cells however this effect was more pronounced in BM 53BP1-positive and BM FANCD2-positive cells at both 100 mGy and 1 Gy doses (γH2AX (p=0.1 and p=0.06), 53BP1 (p=0.002 and p=0.02) and FANCD2 (p=0.02 and p=0.1), respectively).
Conclusion
Our data show that transplacental exposure of human UCB and murine BM cells to carcinogens BQ/HQ and irradiation increases levels of DNA damage presumably leading to increased genome instability. Furthermore, a marked increased phosphorylation of histone H2AX, 53BP1 and FANCD2 in BM cells indicates the effect of a radiation-induced bystander mechanism. DNA damaging secretion conferred by irradiation exposure in the ex vivo model could be prevented by applying nanotechnology-based drug delivery molecules to the placental conditioned medium and further investigations are ongoing. These findings suggest the importance of placenta as a barrier in DNA damage signal propagation during embryo development and provide new insights into the link between placental signaling and foetal leukaemogenesis. Whether these events lead to the accumulation of further genetic aberrations in the context of a minimal two-hit model for leukaemia initiation remains to be further elucidated.

Session topic: E-poster
Keyword(s): Acute leukemia
{{ help_message }}
{{filter}}


