PHASE II STUDY OF CLADRIBINE AND LOW-DOSE ARAC ALTERNATING WITH DECITABINE IN OLDER PATIENTS WITH AML.
(Abstract release date: 05/19/16)
EHA Library. Kadia T. 06/10/16; 133175; P187
Disclosure(s): Research support from Celgene, Bristol Meyers Squibb; Consulting with Novartis, Pfizer;

Dr. Tapan Kadia
Contributions
Contributions
Abstract
Abstract: P187
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Treatment of AML in older and unfit patients (pts) with intensive chemotherapy is complicated by increased toxicity and high early mortality. Additionally, many of these pts are not ideal candidates for stem cell transplant (SCT). Lower intensity approaches using hypomethylating agents (HMAs) are currently used for these pts, but more effective therapies are needed. Cladribine, which itself has hypomethylating properties, has been shown to improve survival in AML in combination with standard-dose araC (Holowiecki JCO 2012).
Aims
We developed a low-intensity, prolonged-maintenance treatment protocol studying the combination of cladribine and low-dose araC (LDAC) alternating with decitabine (DAC) in patients aged ≥ 60.
Methods
Pts with adequate organ function and newly diagnosed AML (excluding APL), including secondary- (s-AML) and therapy-related AML (t-AML), and high risk MDS were eligible. Induction was cladribine 5 mg/m2 IV over 30 minutes on days 1-5 followed by araC 20mg SQ BID on days 1-10. Consolidation/maintenance consisted of 2 cycles of cladribine 5 mg/m2 IV over 30 minutes on days 1-3 + araC 20 mg SQ BID on days 1-10 alternating with 2 cycles of DAC 20 mg/m2 on days 1-5, for a maximum of 18 cycles. One cycle was 4 weeks and up to 2 cycles of induction were allowed.
Results
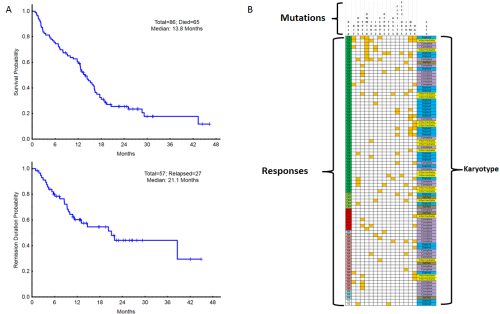
A total of 86 pts have been enrolled with a median age of 69 years (range, 49-85), including 37 pts (43%) ≥ age 70. 49 pts (57%) had s-AML or t-AML and 16 pts (19%) had therapy for an antecedent hematologic disorder. Pt characteristics are listed in Table 1. Of the 86 pts evaluable for response, there were 52 CR (60%), 5 CRp (6%), 2 CRi (2%) and 1 PR (1%) for an overall response rate (CR/CRp/CRi) of 69% (59/86). Of the 49 pts who achieved a CR/CRp and had minimal residual disease (MRD) testing by flow cytometry at day 29, 24 pts (49%), achieved MRD negativity. However, MRD negativity at day 29 did not correlate with improved OS. 13 of the 57 pts (23%) who achieved a CR/CRp went on to SCT. With a median follow-up of 24+ months (m), the median OS is 13.8 m (16.5m in responders) and the median CR duration is 21.1m. (Figure 1a) The 1-year OS estimate is 57%. All pts had cytogenetic and molecular characterization of their AML prior to treatment, allowing correlation with outcomes (Figure 1b). Responses and median OS among different subgroups are shown in Table 1. The 4-week mortality was 1%. Grade ≥3 infections occurred in 16% of pts. There were no treatment-related grade 3/4 non-heme adverse events (AEs). Most common non-hematologic AEs were elevated bilirubin, nausea/vomiting, rash/itching, diarrhea and mucositis.
Conclusion
SummaryThe low intensity program of cladribine + LDAC alternating with DAC produces durable responses and is a well-tolerated ambulatory regimen for older patients, including those with unfavorable-risk features.
Session topic: Acute myeloid leukemia - Clinical 1
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Treatment of AML in older and unfit patients (pts) with intensive chemotherapy is complicated by increased toxicity and high early mortality. Additionally, many of these pts are not ideal candidates for stem cell transplant (SCT). Lower intensity approaches using hypomethylating agents (HMAs) are currently used for these pts, but more effective therapies are needed. Cladribine, which itself has hypomethylating properties, has been shown to improve survival in AML in combination with standard-dose araC (Holowiecki JCO 2012).
Aims
We developed a low-intensity, prolonged-maintenance treatment protocol studying the combination of cladribine and low-dose araC (LDAC) alternating with decitabine (DAC) in patients aged ≥ 60.
Methods
Pts with adequate organ function and newly diagnosed AML (excluding APL), including secondary- (s-AML) and therapy-related AML (t-AML), and high risk MDS were eligible. Induction was cladribine 5 mg/m2 IV over 30 minutes on days 1-5 followed by araC 20mg SQ BID on days 1-10. Consolidation/maintenance consisted of 2 cycles of cladribine 5 mg/m2 IV over 30 minutes on days 1-3 + araC 20 mg SQ BID on days 1-10 alternating with 2 cycles of DAC 20 mg/m2 on days 1-5, for a maximum of 18 cycles. One cycle was 4 weeks and up to 2 cycles of induction were allowed.
Results
A total of 86 pts have been enrolled with a median age of 69 years (range, 49-85), including 37 pts (43%) ≥ age 70. 49 pts (57%) had s-AML or t-AML and 16 pts (19%) had therapy for an antecedent hematologic disorder. Pt characteristics are listed in Table 1. Of the 86 pts evaluable for response, there were 52 CR (60%), 5 CRp (6%), 2 CRi (2%) and 1 PR (1%) for an overall response rate (CR/CRp/CRi) of 69% (59/86). Of the 49 pts who achieved a CR/CRp and had minimal residual disease (MRD) testing by flow cytometry at day 29, 24 pts (49%), achieved MRD negativity. However, MRD negativity at day 29 did not correlate with improved OS. 13 of the 57 pts (23%) who achieved a CR/CRp went on to SCT. With a median follow-up of 24+ months (m), the median OS is 13.8 m (16.5m in responders) and the median CR duration is 21.1m. (Figure 1a) The 1-year OS estimate is 57%. All pts had cytogenetic and molecular characterization of their AML prior to treatment, allowing correlation with outcomes (Figure 1b). Responses and median OS among different subgroups are shown in Table 1. The 4-week mortality was 1%. Grade ≥3 infections occurred in 16% of pts. There were no treatment-related grade 3/4 non-heme adverse events (AEs). Most common non-hematologic AEs were elevated bilirubin, nausea/vomiting, rash/itching, diarrhea and mucositis.
| Table 1. Patient Characteristics (N=74) | ||||
| Characteristic | N (%) | Outcomes by Genetic Subset (N) | CR/CRp | Median OS (m) |
| Median age [Range] | 69 [49-85] | Diploid karyotype (27) | 88% | 16.4 |
| Cytogenetics | Adverse karyotype (35) | 46% | 12.5 | |
| Diploid | 27 (31) | Complex [> 2 abnl] karyotype (23) | 43% | 5.3 |
| Adverse | 35 (41) | TP53 mutated (12) | 50% | 16.2 |
| Misc. other | 18 (21) | DNMT3a mutated (9) | 89% | 12.6 |
| Insufficient | 6 (7) | FLT3-ITD (8) | 100% | 14.2 |
| Median BM Blast [Range] | 32 [8-95] | JAK2 mutated (7) | 57% | 7.5 |
| Median WBC [Range] | 2.7 [0.5-72.3] | NPM1 mutated (14) | 100% | 15.2 |
| Median Platelets [Range] | 41 [4-772] | RAS mutated (18) | 61% | 14.3 |
| Median Creatinine [Range] | 0.89 [0.46-1.94] | |||
| Median Bilirubin [Range] | 0.6 [0.2-2] | |||
Conclusion
SummaryThe low intensity program of cladribine + LDAC alternating with DAC produces durable responses and is a well-tolerated ambulatory regimen for older patients, including those with unfavorable-risk features.

Session topic: Acute myeloid leukemia - Clinical 1
Abstract: P187
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Treatment of AML in older and unfit patients (pts) with intensive chemotherapy is complicated by increased toxicity and high early mortality. Additionally, many of these pts are not ideal candidates for stem cell transplant (SCT). Lower intensity approaches using hypomethylating agents (HMAs) are currently used for these pts, but more effective therapies are needed. Cladribine, which itself has hypomethylating properties, has been shown to improve survival in AML in combination with standard-dose araC (Holowiecki JCO 2012).
Aims
We developed a low-intensity, prolonged-maintenance treatment protocol studying the combination of cladribine and low-dose araC (LDAC) alternating with decitabine (DAC) in patients aged ≥ 60.
Methods
Pts with adequate organ function and newly diagnosed AML (excluding APL), including secondary- (s-AML) and therapy-related AML (t-AML), and high risk MDS were eligible. Induction was cladribine 5 mg/m2 IV over 30 minutes on days 1-5 followed by araC 20mg SQ BID on days 1-10. Consolidation/maintenance consisted of 2 cycles of cladribine 5 mg/m2 IV over 30 minutes on days 1-3 + araC 20 mg SQ BID on days 1-10 alternating with 2 cycles of DAC 20 mg/m2 on days 1-5, for a maximum of 18 cycles. One cycle was 4 weeks and up to 2 cycles of induction were allowed.
Results
A total of 86 pts have been enrolled with a median age of 69 years (range, 49-85), including 37 pts (43%) ≥ age 70. 49 pts (57%) had s-AML or t-AML and 16 pts (19%) had therapy for an antecedent hematologic disorder. Pt characteristics are listed in Table 1. Of the 86 pts evaluable for response, there were 52 CR (60%), 5 CRp (6%), 2 CRi (2%) and 1 PR (1%) for an overall response rate (CR/CRp/CRi) of 69% (59/86). Of the 49 pts who achieved a CR/CRp and had minimal residual disease (MRD) testing by flow cytometry at day 29, 24 pts (49%), achieved MRD negativity. However, MRD negativity at day 29 did not correlate with improved OS. 13 of the 57 pts (23%) who achieved a CR/CRp went on to SCT. With a median follow-up of 24+ months (m), the median OS is 13.8 m (16.5m in responders) and the median CR duration is 21.1m. (Figure 1a) The 1-year OS estimate is 57%. All pts had cytogenetic and molecular characterization of their AML prior to treatment, allowing correlation with outcomes (Figure 1b). Responses and median OS among different subgroups are shown in Table 1. The 4-week mortality was 1%. Grade ≥3 infections occurred in 16% of pts. There were no treatment-related grade 3/4 non-heme adverse events (AEs). Most common non-hematologic AEs were elevated bilirubin, nausea/vomiting, rash/itching, diarrhea and mucositis.
Conclusion
SummaryThe low intensity program of cladribine + LDAC alternating with DAC produces durable responses and is a well-tolerated ambulatory regimen for older patients, including those with unfavorable-risk features.

Session topic: Acute myeloid leukemia - Clinical 1
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Treatment of AML in older and unfit patients (pts) with intensive chemotherapy is complicated by increased toxicity and high early mortality. Additionally, many of these pts are not ideal candidates for stem cell transplant (SCT). Lower intensity approaches using hypomethylating agents (HMAs) are currently used for these pts, but more effective therapies are needed. Cladribine, which itself has hypomethylating properties, has been shown to improve survival in AML in combination with standard-dose araC (Holowiecki JCO 2012).
Aims
We developed a low-intensity, prolonged-maintenance treatment protocol studying the combination of cladribine and low-dose araC (LDAC) alternating with decitabine (DAC) in patients aged ≥ 60.
Methods
Pts with adequate organ function and newly diagnosed AML (excluding APL), including secondary- (s-AML) and therapy-related AML (t-AML), and high risk MDS were eligible. Induction was cladribine 5 mg/m2 IV over 30 minutes on days 1-5 followed by araC 20mg SQ BID on days 1-10. Consolidation/maintenance consisted of 2 cycles of cladribine 5 mg/m2 IV over 30 minutes on days 1-3 + araC 20 mg SQ BID on days 1-10 alternating with 2 cycles of DAC 20 mg/m2 on days 1-5, for a maximum of 18 cycles. One cycle was 4 weeks and up to 2 cycles of induction were allowed.
Results
A total of 86 pts have been enrolled with a median age of 69 years (range, 49-85), including 37 pts (43%) ≥ age 70. 49 pts (57%) had s-AML or t-AML and 16 pts (19%) had therapy for an antecedent hematologic disorder. Pt characteristics are listed in Table 1. Of the 86 pts evaluable for response, there were 52 CR (60%), 5 CRp (6%), 2 CRi (2%) and 1 PR (1%) for an overall response rate (CR/CRp/CRi) of 69% (59/86). Of the 49 pts who achieved a CR/CRp and had minimal residual disease (MRD) testing by flow cytometry at day 29, 24 pts (49%), achieved MRD negativity. However, MRD negativity at day 29 did not correlate with improved OS. 13 of the 57 pts (23%) who achieved a CR/CRp went on to SCT. With a median follow-up of 24+ months (m), the median OS is 13.8 m (16.5m in responders) and the median CR duration is 21.1m. (Figure 1a) The 1-year OS estimate is 57%. All pts had cytogenetic and molecular characterization of their AML prior to treatment, allowing correlation with outcomes (Figure 1b). Responses and median OS among different subgroups are shown in Table 1. The 4-week mortality was 1%. Grade ≥3 infections occurred in 16% of pts. There were no treatment-related grade 3/4 non-heme adverse events (AEs). Most common non-hematologic AEs were elevated bilirubin, nausea/vomiting, rash/itching, diarrhea and mucositis.
| Table 1. Patient Characteristics (N=74) | ||||
| Characteristic | N (%) | Outcomes by Genetic Subset (N) | CR/CRp | Median OS (m) |
| Median age [Range] | 69 [49-85] | Diploid karyotype (27) | 88% | 16.4 |
| Cytogenetics | Adverse karyotype (35) | 46% | 12.5 | |
| Diploid | 27 (31) | Complex [> 2 abnl] karyotype (23) | 43% | 5.3 |
| Adverse | 35 (41) | TP53 mutated (12) | 50% | 16.2 |
| Misc. other | 18 (21) | DNMT3a mutated (9) | 89% | 12.6 |
| Insufficient | 6 (7) | FLT3-ITD (8) | 100% | 14.2 |
| Median BM Blast [Range] | 32 [8-95] | JAK2 mutated (7) | 57% | 7.5 |
| Median WBC [Range] | 2.7 [0.5-72.3] | NPM1 mutated (14) | 100% | 15.2 |
| Median Platelets [Range] | 41 [4-772] | RAS mutated (18) | 61% | 14.3 |
| Median Creatinine [Range] | 0.89 [0.46-1.94] | |||
| Median Bilirubin [Range] | 0.6 [0.2-2] | |||
Conclusion
SummaryThe low intensity program of cladribine + LDAC alternating with DAC produces durable responses and is a well-tolerated ambulatory regimen for older patients, including those with unfavorable-risk features.

Session topic: Acute myeloid leukemia - Clinical 1
{{ help_message }}
{{filter}}


