ORAL THROMBOPOIETIN RECEPTOR AGONIST ELTROMBOPAG TREATMENT DURING INDUCTION CHEMOTHERAPY FOR ACUTE MYELOGENOUS LEUKEMIA (AML): RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PHASE 2 STUDY
(Abstract release date: 05/19/16)
EHA Library. Frey N. 06/10/16; 133173; P185

Noëlle Frey
Contributions
Contributions
Abstract
Abstract: P185
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Thrombocytopenia is a significant problem for patients with AML due to the disease process and the side effects of its treatment. Eltrombopag is an orally bioavailable, small molecule, thrombopoietin receptor agonist that stimulates platelet production by a mechanism similar but not identical to endogenous thrombopoietin. A randomized, placebo-controlled, Phase 1 study suggested a benefit of eltrombopag treatment of up to 300 mg daily in patients with advanced myelodysplastic syndromes or AML (Platzbecker U, et al. Lancet Haematol. 2015;2:e417-26).
Aims
To assess the safety and tolerability of daily treatment with eltrombopag versus placebo in patients with AML receiving anthracycline-based induction treatment; these included rates of adverse events (AEs), changes in left ventricular ejection fraction (LVEF), and clinical laboratory parameters including effects on cytopenias.
Methods
In this randomized, double-blind, Phase 2 study, adult patients with AML of any subtype (except M3 and M7) (n=148), and who had not received previous treatment for AML, received standard induction chemotherapy with daunorubicin on Days 1–3 and cytarabine on Days 1–7 (Table), and eltrombopag 200 mg (100 mg for East Asians) or placebo daily, starting on Day 4. Study drug could be titrated to 100–300 mg based on platelet counts. Randomization was stratified by antecedent malignant hematologic disorder (yes/no) and age (18–60 vs >60 years). Safety was the primary endpoint. The trial began in September 2013, enrollment is completed, and all patients completed induction/reinduction. Follow up for survival continues. This primary analysis represents results through June 2015.
Results
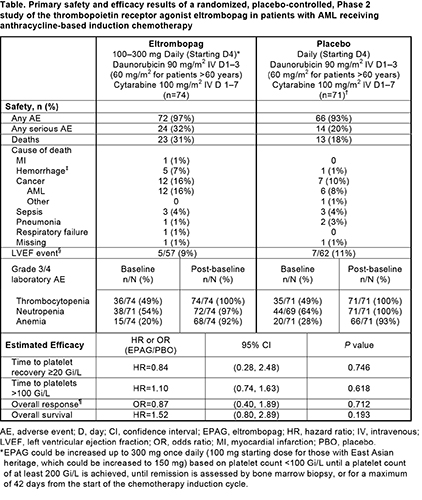
The eltrombopag versus placebo groups, respectively, were well matched at baseline: overall median (range) age 58.5 (23–77) versus 59.5 (21–75); median (range) platelet counts 51.5 (5–241) Gi/L versus 50.0 (9–232) Gi/L; 51% versus 42% females; and 22% poor karyotype in both groups. The frequency of AEs did not differ between treatment groups, but frequencies of serious AEs and deaths were greater in the eltrombopag group (with more deaths due to hemorrhage and cancer, which included disease under study and other malignancies) (Table). Deaths due to hemorrhage included pulmonary and intracranial/cerebral/brain hemorrhages, and occured during periods of thrombocytopenia. Thromboembolic events were similar between groups (7% vs 6% for eltrombopag vs placebo). Reductions in LVEF (a potential effect of drug-drug interaction between eltrombopag and daunorubicin) did not differ between groups (Table). Increases from baseline in Grade 3/4 laboratory AEs for thrombocytopenia, neutropenia, or anemia did not differ significantly between treatment groups. Time to platelet recovery, time to platelets >100 Gi/L, overall disease response, and survival also did not differ significantly between groups (Table).
Conclusion
The thrombopoietin receptor agonist eltrombopag did not significantly affect rates of thrombocytopenia during induction therapy with daunorubicin and cytarabine or disease response in adults with AML compared with placebo in this primary analysis. No statistically significant difference in LVEF events between eltrombopag and placebo was observed.Funding: This study (NCT01890746) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.
Session topic: Acute myeloid leukemia - Clinical 1
Keyword(s): AML, Cytarabine, Thrombocytopenia
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Thrombocytopenia is a significant problem for patients with AML due to the disease process and the side effects of its treatment. Eltrombopag is an orally bioavailable, small molecule, thrombopoietin receptor agonist that stimulates platelet production by a mechanism similar but not identical to endogenous thrombopoietin. A randomized, placebo-controlled, Phase 1 study suggested a benefit of eltrombopag treatment of up to 300 mg daily in patients with advanced myelodysplastic syndromes or AML (Platzbecker U, et al. Lancet Haematol. 2015;2:e417-26).
Aims
To assess the safety and tolerability of daily treatment with eltrombopag versus placebo in patients with AML receiving anthracycline-based induction treatment; these included rates of adverse events (AEs), changes in left ventricular ejection fraction (LVEF), and clinical laboratory parameters including effects on cytopenias.
Methods
In this randomized, double-blind, Phase 2 study, adult patients with AML of any subtype (except M3 and M7) (n=148), and who had not received previous treatment for AML, received standard induction chemotherapy with daunorubicin on Days 1–3 and cytarabine on Days 1–7 (Table), and eltrombopag 200 mg (100 mg for East Asians) or placebo daily, starting on Day 4. Study drug could be titrated to 100–300 mg based on platelet counts. Randomization was stratified by antecedent malignant hematologic disorder (yes/no) and age (18–60 vs >60 years). Safety was the primary endpoint. The trial began in September 2013, enrollment is completed, and all patients completed induction/reinduction. Follow up for survival continues. This primary analysis represents results through June 2015.
Results
The eltrombopag versus placebo groups, respectively, were well matched at baseline: overall median (range) age 58.5 (23–77) versus 59.5 (21–75); median (range) platelet counts 51.5 (5–241) Gi/L versus 50.0 (9–232) Gi/L; 51% versus 42% females; and 22% poor karyotype in both groups. The frequency of AEs did not differ between treatment groups, but frequencies of serious AEs and deaths were greater in the eltrombopag group (with more deaths due to hemorrhage and cancer, which included disease under study and other malignancies) (Table). Deaths due to hemorrhage included pulmonary and intracranial/cerebral/brain hemorrhages, and occured during periods of thrombocytopenia. Thromboembolic events were similar between groups (7% vs 6% for eltrombopag vs placebo). Reductions in LVEF (a potential effect of drug-drug interaction between eltrombopag and daunorubicin) did not differ between groups (Table). Increases from baseline in Grade 3/4 laboratory AEs for thrombocytopenia, neutropenia, or anemia did not differ significantly between treatment groups. Time to platelet recovery, time to platelets >100 Gi/L, overall disease response, and survival also did not differ significantly between groups (Table).
Conclusion
The thrombopoietin receptor agonist eltrombopag did not significantly affect rates of thrombocytopenia during induction therapy with daunorubicin and cytarabine or disease response in adults with AML compared with placebo in this primary analysis. No statistically significant difference in LVEF events between eltrombopag and placebo was observed.Funding: This study (NCT01890746) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.

Session topic: Acute myeloid leukemia - Clinical 1
Keyword(s): AML, Cytarabine, Thrombocytopenia
Abstract: P185
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Thrombocytopenia is a significant problem for patients with AML due to the disease process and the side effects of its treatment. Eltrombopag is an orally bioavailable, small molecule, thrombopoietin receptor agonist that stimulates platelet production by a mechanism similar but not identical to endogenous thrombopoietin. A randomized, placebo-controlled, Phase 1 study suggested a benefit of eltrombopag treatment of up to 300 mg daily in patients with advanced myelodysplastic syndromes or AML (Platzbecker U, et al. Lancet Haematol. 2015;2:e417-26).
Aims
To assess the safety and tolerability of daily treatment with eltrombopag versus placebo in patients with AML receiving anthracycline-based induction treatment; these included rates of adverse events (AEs), changes in left ventricular ejection fraction (LVEF), and clinical laboratory parameters including effects on cytopenias.
Methods
In this randomized, double-blind, Phase 2 study, adult patients with AML of any subtype (except M3 and M7) (n=148), and who had not received previous treatment for AML, received standard induction chemotherapy with daunorubicin on Days 1–3 and cytarabine on Days 1–7 (Table), and eltrombopag 200 mg (100 mg for East Asians) or placebo daily, starting on Day 4. Study drug could be titrated to 100–300 mg based on platelet counts. Randomization was stratified by antecedent malignant hematologic disorder (yes/no) and age (18–60 vs >60 years). Safety was the primary endpoint. The trial began in September 2013, enrollment is completed, and all patients completed induction/reinduction. Follow up for survival continues. This primary analysis represents results through June 2015.
Results
The eltrombopag versus placebo groups, respectively, were well matched at baseline: overall median (range) age 58.5 (23–77) versus 59.5 (21–75); median (range) platelet counts 51.5 (5–241) Gi/L versus 50.0 (9–232) Gi/L; 51% versus 42% females; and 22% poor karyotype in both groups. The frequency of AEs did not differ between treatment groups, but frequencies of serious AEs and deaths were greater in the eltrombopag group (with more deaths due to hemorrhage and cancer, which included disease under study and other malignancies) (Table). Deaths due to hemorrhage included pulmonary and intracranial/cerebral/brain hemorrhages, and occured during periods of thrombocytopenia. Thromboembolic events were similar between groups (7% vs 6% for eltrombopag vs placebo). Reductions in LVEF (a potential effect of drug-drug interaction between eltrombopag and daunorubicin) did not differ between groups (Table). Increases from baseline in Grade 3/4 laboratory AEs for thrombocytopenia, neutropenia, or anemia did not differ significantly between treatment groups. Time to platelet recovery, time to platelets >100 Gi/L, overall disease response, and survival also did not differ significantly between groups (Table).
Conclusion
The thrombopoietin receptor agonist eltrombopag did not significantly affect rates of thrombocytopenia during induction therapy with daunorubicin and cytarabine or disease response in adults with AML compared with placebo in this primary analysis. No statistically significant difference in LVEF events between eltrombopag and placebo was observed.Funding: This study (NCT01890746) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.

Session topic: Acute myeloid leukemia - Clinical 1
Keyword(s): AML, Cytarabine, Thrombocytopenia
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Thrombocytopenia is a significant problem for patients with AML due to the disease process and the side effects of its treatment. Eltrombopag is an orally bioavailable, small molecule, thrombopoietin receptor agonist that stimulates platelet production by a mechanism similar but not identical to endogenous thrombopoietin. A randomized, placebo-controlled, Phase 1 study suggested a benefit of eltrombopag treatment of up to 300 mg daily in patients with advanced myelodysplastic syndromes or AML (Platzbecker U, et al. Lancet Haematol. 2015;2:e417-26).
Aims
To assess the safety and tolerability of daily treatment with eltrombopag versus placebo in patients with AML receiving anthracycline-based induction treatment; these included rates of adverse events (AEs), changes in left ventricular ejection fraction (LVEF), and clinical laboratory parameters including effects on cytopenias.
Methods
In this randomized, double-blind, Phase 2 study, adult patients with AML of any subtype (except M3 and M7) (n=148), and who had not received previous treatment for AML, received standard induction chemotherapy with daunorubicin on Days 1–3 and cytarabine on Days 1–7 (Table), and eltrombopag 200 mg (100 mg for East Asians) or placebo daily, starting on Day 4. Study drug could be titrated to 100–300 mg based on platelet counts. Randomization was stratified by antecedent malignant hematologic disorder (yes/no) and age (18–60 vs >60 years). Safety was the primary endpoint. The trial began in September 2013, enrollment is completed, and all patients completed induction/reinduction. Follow up for survival continues. This primary analysis represents results through June 2015.
Results
The eltrombopag versus placebo groups, respectively, were well matched at baseline: overall median (range) age 58.5 (23–77) versus 59.5 (21–75); median (range) platelet counts 51.5 (5–241) Gi/L versus 50.0 (9–232) Gi/L; 51% versus 42% females; and 22% poor karyotype in both groups. The frequency of AEs did not differ between treatment groups, but frequencies of serious AEs and deaths were greater in the eltrombopag group (with more deaths due to hemorrhage and cancer, which included disease under study and other malignancies) (Table). Deaths due to hemorrhage included pulmonary and intracranial/cerebral/brain hemorrhages, and occured during periods of thrombocytopenia. Thromboembolic events were similar between groups (7% vs 6% for eltrombopag vs placebo). Reductions in LVEF (a potential effect of drug-drug interaction between eltrombopag and daunorubicin) did not differ between groups (Table). Increases from baseline in Grade 3/4 laboratory AEs for thrombocytopenia, neutropenia, or anemia did not differ significantly between treatment groups. Time to platelet recovery, time to platelets >100 Gi/L, overall disease response, and survival also did not differ significantly between groups (Table).
Conclusion
The thrombopoietin receptor agonist eltrombopag did not significantly affect rates of thrombocytopenia during induction therapy with daunorubicin and cytarabine or disease response in adults with AML compared with placebo in this primary analysis. No statistically significant difference in LVEF events between eltrombopag and placebo was observed.Funding: This study (NCT01890746) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.

Session topic: Acute myeloid leukemia - Clinical 1
Keyword(s): AML, Cytarabine, Thrombocytopenia
{{ help_message }}
{{filter}}


