THE COMBINATION OF BORTEZOMIB AND CHEMOTHERAPY IS AN EFFECTIVE TREATMENT TO RE-INDUCE REMISSION IN RELAPSED/REFRACTORY B-CELL PRECURSOR OR T-CELL ACUTE LYMPHOBLASTIC LEUKEMIA
(Abstract release date: 05/19/16)
EHA Library. Bertaina A. 06/10/16; 133156; P168
Disclosure(s): There is no conflict of interest to disclose

Dr. Alice Bertaina
Contributions
Contributions
Abstract
Abstract: P168
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Achievement of a new complete remission (CR) in relapsed/refractory pediatric acute lymphoblastic leukemia (ALL) is hampered by a low response rate and high toxicity, especially after second or subsequent relapses. Bortezomib, the first proteasome inhibitor approved by Food and Drug Administration for multiple myeloma and relapsed non-Hodgkin lymphoma, showed preclinical activity against ALL blasts. Moreover, a phase II study reported by the Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) study group demonstrated that the combination of bortezomib with vincristine, dexamethasone, pegylated asparaginase and doxorubicin carried acceptable toxicity and good efficacy in patients with relapsed B-cell precursor ALL who failed 2-3 previous regimens (Messinger, Blood 2012).
Aims
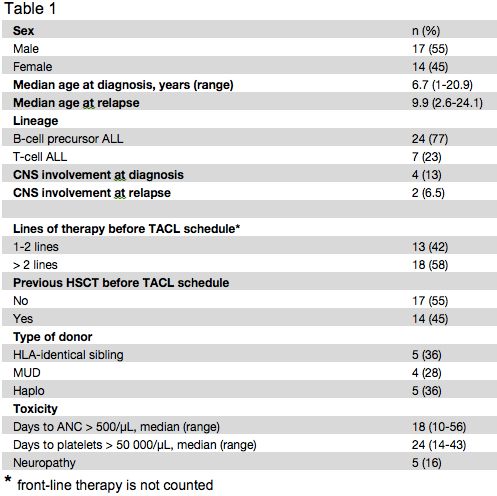
In order to improve the CR rate of children with relapsed/refractory ALL, we evaluated the use of bortezomib in combination with vincristine, dexamethasone, pegylated asparaginase and doxorubicin, as a salvage/re-induction treatment in 24 and 7 children with B-cell precursor and T-cell ALL (2 ETP), respectively. Patient characteristics are reported in Table I.
Methods
Bortezomib (1.3 mg/m2/dose) was administered intravenously on day 1, 4, 8, and 11. Dexamethasone 10 mg/m2/day was given orally for 14 consecutive days. Doxorubicin 60 mg/m2 was given intravenously on day 1. Vincristine 1.5 mg/m2/dose (2 mg maximum dose) was administered intravenously on day 1, 8, 15, and 22. Pegylated asparaginase 2500 units/m2/dose was given intravenously weekly for 4 doses. All patients received intrathecal (IT) cytarabine on day 1. Patients without CNS involvement were given IT methotrexate on day 15. Patients with CNS involvement at time of treatment were given IT methotrexate, methylprednisolone, and cytarabine on day 8, 15, and 22.
Results
Between February 2012 and July 2015, 24 pediatric patients with relapsed B-cell precursor and 7 with T-cell ALL were given the treatment detailed above at the Hematology/Oncology Department of the IRCCS Bambino Gesù Children’s Hospital, Rome, Italy. Seventeen patients were males and 14 females, median age at diagnosis and at time of last leukemia relapse being 6.7 years (range 1-20.9) and 9.9 years (2.6-24.1), respectively. Four patients were CNS-positive at diagnosis, while 4/31 experienced a combined bone marrow (BM) and extramedullary relapse (2 CNS, 1 kidney and 1 gut, respectively). Fourteen children had previously received allogeneic hematopoietic stem cell transplantation (HSCT, see table 1 for details on the donor). Grade 3 (CTCAE v4.03) peripheral motor and sensory neuropathy developed in 5 (16%) patients. Six out of the 31 patients (19.5%) experienced severe infections (2 sepsis due to Geotrycum, 3 to Candida and 1 to multidrug resistant Klebsiella Pneumoniae). Despite the introduction of broad-spectrum antibacterial and antifungal therapy, 4 of them (13%) died (2 each with Geotrycum and Candida infection) without recovering neutrophils. Twenty patients achieved CR (10 with a MRD<0.1%), this leading to a 64.5% overall response rate. Interestingly, in our cohort, not only B-cell precursor patients had a good outcome (15/24, 62.5% CR rate), but also T-cell ALL responded well to this combination of drugs (5/7, 71.4% CR rate). Fifteen of these patients had a long-lasting CR allowing them to receive HSCT resulting in a 2-year Overall Survival (OS) of 37.1% (SE 13.2).
Conclusion
In conclusion, the regimen of bortezomib combined with a 4-drug (vincristine dexamethasone, pegylated asparaginase and doxorubicin) re-induction therapy is effective for children with relapsed/refractory B-cell precursor or T-cell ALL. Considering the risk of life-threatening/fatal infections in a such vulnerable population, an intensive antibacterial and antifungal prophylaxis is strongly recommended.
Session topic: Acute lymphoblastic leukemia - Clinical 1
Keyword(s): Chemotherapy, Proteasome inhibitor, Relapsed acute lymphoblastic leukemia, Remission
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Achievement of a new complete remission (CR) in relapsed/refractory pediatric acute lymphoblastic leukemia (ALL) is hampered by a low response rate and high toxicity, especially after second or subsequent relapses. Bortezomib, the first proteasome inhibitor approved by Food and Drug Administration for multiple myeloma and relapsed non-Hodgkin lymphoma, showed preclinical activity against ALL blasts. Moreover, a phase II study reported by the Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) study group demonstrated that the combination of bortezomib with vincristine, dexamethasone, pegylated asparaginase and doxorubicin carried acceptable toxicity and good efficacy in patients with relapsed B-cell precursor ALL who failed 2-3 previous regimens (Messinger, Blood 2012).
Aims
In order to improve the CR rate of children with relapsed/refractory ALL, we evaluated the use of bortezomib in combination with vincristine, dexamethasone, pegylated asparaginase and doxorubicin, as a salvage/re-induction treatment in 24 and 7 children with B-cell precursor and T-cell ALL (2 ETP), respectively. Patient characteristics are reported in Table I.
Methods
Bortezomib (1.3 mg/m2/dose) was administered intravenously on day 1, 4, 8, and 11. Dexamethasone 10 mg/m2/day was given orally for 14 consecutive days. Doxorubicin 60 mg/m2 was given intravenously on day 1. Vincristine 1.5 mg/m2/dose (2 mg maximum dose) was administered intravenously on day 1, 8, 15, and 22. Pegylated asparaginase 2500 units/m2/dose was given intravenously weekly for 4 doses. All patients received intrathecal (IT) cytarabine on day 1. Patients without CNS involvement were given IT methotrexate on day 15. Patients with CNS involvement at time of treatment were given IT methotrexate, methylprednisolone, and cytarabine on day 8, 15, and 22.
Results
Between February 2012 and July 2015, 24 pediatric patients with relapsed B-cell precursor and 7 with T-cell ALL were given the treatment detailed above at the Hematology/Oncology Department of the IRCCS Bambino Gesù Children’s Hospital, Rome, Italy. Seventeen patients were males and 14 females, median age at diagnosis and at time of last leukemia relapse being 6.7 years (range 1-20.9) and 9.9 years (2.6-24.1), respectively. Four patients were CNS-positive at diagnosis, while 4/31 experienced a combined bone marrow (BM) and extramedullary relapse (2 CNS, 1 kidney and 1 gut, respectively). Fourteen children had previously received allogeneic hematopoietic stem cell transplantation (HSCT, see table 1 for details on the donor). Grade 3 (CTCAE v4.03) peripheral motor and sensory neuropathy developed in 5 (16%) patients. Six out of the 31 patients (19.5%) experienced severe infections (2 sepsis due to Geotrycum, 3 to Candida and 1 to multidrug resistant Klebsiella Pneumoniae). Despite the introduction of broad-spectrum antibacterial and antifungal therapy, 4 of them (13%) died (2 each with Geotrycum and Candida infection) without recovering neutrophils. Twenty patients achieved CR (10 with a MRD<0.1%), this leading to a 64.5% overall response rate. Interestingly, in our cohort, not only B-cell precursor patients had a good outcome (15/24, 62.5% CR rate), but also T-cell ALL responded well to this combination of drugs (5/7, 71.4% CR rate). Fifteen of these patients had a long-lasting CR allowing them to receive HSCT resulting in a 2-year Overall Survival (OS) of 37.1% (SE 13.2).
Conclusion
In conclusion, the regimen of bortezomib combined with a 4-drug (vincristine dexamethasone, pegylated asparaginase and doxorubicin) re-induction therapy is effective for children with relapsed/refractory B-cell precursor or T-cell ALL. Considering the risk of life-threatening/fatal infections in a such vulnerable population, an intensive antibacterial and antifungal prophylaxis is strongly recommended.

Session topic: Acute lymphoblastic leukemia - Clinical 1
Keyword(s): Chemotherapy, Proteasome inhibitor, Relapsed acute lymphoblastic leukemia, Remission
Abstract: P168
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Achievement of a new complete remission (CR) in relapsed/refractory pediatric acute lymphoblastic leukemia (ALL) is hampered by a low response rate and high toxicity, especially after second or subsequent relapses. Bortezomib, the first proteasome inhibitor approved by Food and Drug Administration for multiple myeloma and relapsed non-Hodgkin lymphoma, showed preclinical activity against ALL blasts. Moreover, a phase II study reported by the Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) study group demonstrated that the combination of bortezomib with vincristine, dexamethasone, pegylated asparaginase and doxorubicin carried acceptable toxicity and good efficacy in patients with relapsed B-cell precursor ALL who failed 2-3 previous regimens (Messinger, Blood 2012).
Aims
In order to improve the CR rate of children with relapsed/refractory ALL, we evaluated the use of bortezomib in combination with vincristine, dexamethasone, pegylated asparaginase and doxorubicin, as a salvage/re-induction treatment in 24 and 7 children with B-cell precursor and T-cell ALL (2 ETP), respectively. Patient characteristics are reported in Table I.
Methods
Bortezomib (1.3 mg/m2/dose) was administered intravenously on day 1, 4, 8, and 11. Dexamethasone 10 mg/m2/day was given orally for 14 consecutive days. Doxorubicin 60 mg/m2 was given intravenously on day 1. Vincristine 1.5 mg/m2/dose (2 mg maximum dose) was administered intravenously on day 1, 8, 15, and 22. Pegylated asparaginase 2500 units/m2/dose was given intravenously weekly for 4 doses. All patients received intrathecal (IT) cytarabine on day 1. Patients without CNS involvement were given IT methotrexate on day 15. Patients with CNS involvement at time of treatment were given IT methotrexate, methylprednisolone, and cytarabine on day 8, 15, and 22.
Results
Between February 2012 and July 2015, 24 pediatric patients with relapsed B-cell precursor and 7 with T-cell ALL were given the treatment detailed above at the Hematology/Oncology Department of the IRCCS Bambino Gesù Children’s Hospital, Rome, Italy. Seventeen patients were males and 14 females, median age at diagnosis and at time of last leukemia relapse being 6.7 years (range 1-20.9) and 9.9 years (2.6-24.1), respectively. Four patients were CNS-positive at diagnosis, while 4/31 experienced a combined bone marrow (BM) and extramedullary relapse (2 CNS, 1 kidney and 1 gut, respectively). Fourteen children had previously received allogeneic hematopoietic stem cell transplantation (HSCT, see table 1 for details on the donor). Grade 3 (CTCAE v4.03) peripheral motor and sensory neuropathy developed in 5 (16%) patients. Six out of the 31 patients (19.5%) experienced severe infections (2 sepsis due to Geotrycum, 3 to Candida and 1 to multidrug resistant Klebsiella Pneumoniae). Despite the introduction of broad-spectrum antibacterial and antifungal therapy, 4 of them (13%) died (2 each with Geotrycum and Candida infection) without recovering neutrophils. Twenty patients achieved CR (10 with a MRD<0.1%), this leading to a 64.5% overall response rate. Interestingly, in our cohort, not only B-cell precursor patients had a good outcome (15/24, 62.5% CR rate), but also T-cell ALL responded well to this combination of drugs (5/7, 71.4% CR rate). Fifteen of these patients had a long-lasting CR allowing them to receive HSCT resulting in a 2-year Overall Survival (OS) of 37.1% (SE 13.2).
Conclusion
In conclusion, the regimen of bortezomib combined with a 4-drug (vincristine dexamethasone, pegylated asparaginase and doxorubicin) re-induction therapy is effective for children with relapsed/refractory B-cell precursor or T-cell ALL. Considering the risk of life-threatening/fatal infections in a such vulnerable population, an intensive antibacterial and antifungal prophylaxis is strongly recommended.

Session topic: Acute lymphoblastic leukemia - Clinical 1
Keyword(s): Chemotherapy, Proteasome inhibitor, Relapsed acute lymphoblastic leukemia, Remission
Type: Poster Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 17:15 - 18:45
Location: Poster area (Hall H)
Background
Achievement of a new complete remission (CR) in relapsed/refractory pediatric acute lymphoblastic leukemia (ALL) is hampered by a low response rate and high toxicity, especially after second or subsequent relapses. Bortezomib, the first proteasome inhibitor approved by Food and Drug Administration for multiple myeloma and relapsed non-Hodgkin lymphoma, showed preclinical activity against ALL blasts. Moreover, a phase II study reported by the Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) study group demonstrated that the combination of bortezomib with vincristine, dexamethasone, pegylated asparaginase and doxorubicin carried acceptable toxicity and good efficacy in patients with relapsed B-cell precursor ALL who failed 2-3 previous regimens (Messinger, Blood 2012).
Aims
In order to improve the CR rate of children with relapsed/refractory ALL, we evaluated the use of bortezomib in combination with vincristine, dexamethasone, pegylated asparaginase and doxorubicin, as a salvage/re-induction treatment in 24 and 7 children with B-cell precursor and T-cell ALL (2 ETP), respectively. Patient characteristics are reported in Table I.
Methods
Bortezomib (1.3 mg/m2/dose) was administered intravenously on day 1, 4, 8, and 11. Dexamethasone 10 mg/m2/day was given orally for 14 consecutive days. Doxorubicin 60 mg/m2 was given intravenously on day 1. Vincristine 1.5 mg/m2/dose (2 mg maximum dose) was administered intravenously on day 1, 8, 15, and 22. Pegylated asparaginase 2500 units/m2/dose was given intravenously weekly for 4 doses. All patients received intrathecal (IT) cytarabine on day 1. Patients without CNS involvement were given IT methotrexate on day 15. Patients with CNS involvement at time of treatment were given IT methotrexate, methylprednisolone, and cytarabine on day 8, 15, and 22.
Results
Between February 2012 and July 2015, 24 pediatric patients with relapsed B-cell precursor and 7 with T-cell ALL were given the treatment detailed above at the Hematology/Oncology Department of the IRCCS Bambino Gesù Children’s Hospital, Rome, Italy. Seventeen patients were males and 14 females, median age at diagnosis and at time of last leukemia relapse being 6.7 years (range 1-20.9) and 9.9 years (2.6-24.1), respectively. Four patients were CNS-positive at diagnosis, while 4/31 experienced a combined bone marrow (BM) and extramedullary relapse (2 CNS, 1 kidney and 1 gut, respectively). Fourteen children had previously received allogeneic hematopoietic stem cell transplantation (HSCT, see table 1 for details on the donor). Grade 3 (CTCAE v4.03) peripheral motor and sensory neuropathy developed in 5 (16%) patients. Six out of the 31 patients (19.5%) experienced severe infections (2 sepsis due to Geotrycum, 3 to Candida and 1 to multidrug resistant Klebsiella Pneumoniae). Despite the introduction of broad-spectrum antibacterial and antifungal therapy, 4 of them (13%) died (2 each with Geotrycum and Candida infection) without recovering neutrophils. Twenty patients achieved CR (10 with a MRD<0.1%), this leading to a 64.5% overall response rate. Interestingly, in our cohort, not only B-cell precursor patients had a good outcome (15/24, 62.5% CR rate), but also T-cell ALL responded well to this combination of drugs (5/7, 71.4% CR rate). Fifteen of these patients had a long-lasting CR allowing them to receive HSCT resulting in a 2-year Overall Survival (OS) of 37.1% (SE 13.2).
Conclusion
In conclusion, the regimen of bortezomib combined with a 4-drug (vincristine dexamethasone, pegylated asparaginase and doxorubicin) re-induction therapy is effective for children with relapsed/refractory B-cell precursor or T-cell ALL. Considering the risk of life-threatening/fatal infections in a such vulnerable population, an intensive antibacterial and antifungal prophylaxis is strongly recommended.

Session topic: Acute lymphoblastic leukemia - Clinical 1
Keyword(s): Chemotherapy, Proteasome inhibitor, Relapsed acute lymphoblastic leukemia, Remission
{{ help_message }}
{{filter}}


