IMPACT ON MOTHER AND NEWBORN OF ANTITHROMBIN III DEFICIENCY DURING PREGNANCY.
(Abstract release date: 05/19/16)
EHA Library. Fernandez-Jimenez D. 06/09/16; 133113; E1564

Mrs. Dolores Fernandez-Jimenez
Contributions
Contributions
Abstract
Abstract: E1564
Type: Eposter Presentation
Background
During pregnancy, the risk of suffering thromboembolic disease increases fivefold. The most significant risk factors are a prior history of thrombosis followed by the presence of thrombophilia, with ATIII deficiency associated to a greater risk of thrombosis and complications during pregnancy (including miscarriage, intrauterine growth restriction, fetal death, placental abruption, pre-eclampsia and HELLP syndrome). The ideal treatment for such women remains undefined, given that limited clinical data is available and most cases are reported on an individual basis or on a small scale. It is widely accepted that most of these patients should receive thromboembolic prophylaxis, although the dosage, duration and type of anticoagulant remains a contentious issue.
Aims
Evaluate the impact of antithrombin deficiency and the different treatments in pregnant women.
Methods
Retrospective 30-year study of pregnant women with ATIII deficiency. We analysed a total of 57 pregnancies in 21 women diagnosed with ATIII deficiency (10 due to family history, 2 due to a child having shown the deficiency and the remainder due to personal history). The average age was 29 (19-41). 4 pregnant women displayed cardiovascular risk factors (1 high blood pressure, 1 smoker, 1 dyslipidemia and the other a smoker plus sufferer of dyslipidemia) and 8 had additional thrombophilia (6 with heterozygous Factor V Leiden, one protein S deficiency and one combined homozygous factor XII deficiency and heterozygous Factor V Leiden).
Results
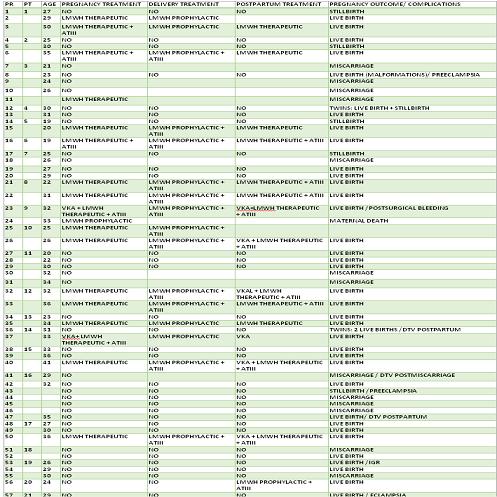
Of the 57 pregnant women, 2 of whom were carrying twins, 38 received no treatment as the antithrombin III deficiency was diagnosed at a later date. From this group, 22 resulted in live births, 12 miscarried, 6 suffered an intra-uterine stillbirth and 2 experienced intrauterine growth retardation. In terms of maternal complications that went untreated, 2 women developed pre-eclampsia and there were 5 thrombotic events (2 pulmonary thromboembolisms, one of which with sinus-related thrombosis and 4 deep vein thromboses). As part of 6 pregnancies, patients received Acenocumarol due to a history of thromboses.19 pregnant women received treatment: 15 low molecular weight heparin (LMWH) in therapeutic doses subject to Anti Xa control (6 received Acenocumarol previously due to a history of thromboses), 1 LMWH in intermediate doses and 3 in prophylactic doses. At the same time, ATIII 50UI/kg/72 hours was administered to 4 patients and 2 took Acenocumarol from week 12. During the peripartum period, two received LMWH, another ATIII and 13 ATIII linked to LMWH in prophylactic doses. Post-partum, 9 patients received Acenocumarol (6 having received bridging treatment via ATIII and LMWH and 3 just via LMWH) and 10 LMWH (5 of which with ATIII).From amongst the 19 pregnancies as part of which treatment was administered, 17 resulted in live births. Complications recorded included one pre-eclampsia, one miscarriage and one maternal death due to severe sinus thrombosis, all three of which occurred in patients receiving a prophylactic dose of Heparin. The only adverse effect of the treatment involved bleeding from a caesarean wound in one of the patients receiving ATIII and LMWH. 10 received an epidural with no adverse effects.
Conclusion
In our experience, the rate of maternal-fetal complications in patients with an ATIII deficiency is clearly higher than amongst the general population; furthermore, there is a higher number of adverse effects in the group receiving no treatment. Although in the series of cases in hand, therapy using LMWH and/or ATIII has been demonstrated as an effective and safe strategy, these patients must be subject to strict monitoring, as complications can occur throughout the pregnancy and post-partum period, particularly in the case of inadequate thromboprophylaxis. Further research is needed to establish and standardise action protocols.
Session topic: E-poster
Keyword(s): Antithrombin, Pregnancy
Type: Eposter Presentation
Background
During pregnancy, the risk of suffering thromboembolic disease increases fivefold. The most significant risk factors are a prior history of thrombosis followed by the presence of thrombophilia, with ATIII deficiency associated to a greater risk of thrombosis and complications during pregnancy (including miscarriage, intrauterine growth restriction, fetal death, placental abruption, pre-eclampsia and HELLP syndrome). The ideal treatment for such women remains undefined, given that limited clinical data is available and most cases are reported on an individual basis or on a small scale. It is widely accepted that most of these patients should receive thromboembolic prophylaxis, although the dosage, duration and type of anticoagulant remains a contentious issue.
Aims
Evaluate the impact of antithrombin deficiency and the different treatments in pregnant women.
Methods
Retrospective 30-year study of pregnant women with ATIII deficiency. We analysed a total of 57 pregnancies in 21 women diagnosed with ATIII deficiency (10 due to family history, 2 due to a child having shown the deficiency and the remainder due to personal history). The average age was 29 (19-41). 4 pregnant women displayed cardiovascular risk factors (1 high blood pressure, 1 smoker, 1 dyslipidemia and the other a smoker plus sufferer of dyslipidemia) and 8 had additional thrombophilia (6 with heterozygous Factor V Leiden, one protein S deficiency and one combined homozygous factor XII deficiency and heterozygous Factor V Leiden).
Results
Of the 57 pregnant women, 2 of whom were carrying twins, 38 received no treatment as the antithrombin III deficiency was diagnosed at a later date. From this group, 22 resulted in live births, 12 miscarried, 6 suffered an intra-uterine stillbirth and 2 experienced intrauterine growth retardation. In terms of maternal complications that went untreated, 2 women developed pre-eclampsia and there were 5 thrombotic events (2 pulmonary thromboembolisms, one of which with sinus-related thrombosis and 4 deep vein thromboses). As part of 6 pregnancies, patients received Acenocumarol due to a history of thromboses.19 pregnant women received treatment: 15 low molecular weight heparin (LMWH) in therapeutic doses subject to Anti Xa control (6 received Acenocumarol previously due to a history of thromboses), 1 LMWH in intermediate doses and 3 in prophylactic doses. At the same time, ATIII 50UI/kg/72 hours was administered to 4 patients and 2 took Acenocumarol from week 12. During the peripartum period, two received LMWH, another ATIII and 13 ATIII linked to LMWH in prophylactic doses. Post-partum, 9 patients received Acenocumarol (6 having received bridging treatment via ATIII and LMWH and 3 just via LMWH) and 10 LMWH (5 of which with ATIII).From amongst the 19 pregnancies as part of which treatment was administered, 17 resulted in live births. Complications recorded included one pre-eclampsia, one miscarriage and one maternal death due to severe sinus thrombosis, all three of which occurred in patients receiving a prophylactic dose of Heparin. The only adverse effect of the treatment involved bleeding from a caesarean wound in one of the patients receiving ATIII and LMWH. 10 received an epidural with no adverse effects.
Conclusion
In our experience, the rate of maternal-fetal complications in patients with an ATIII deficiency is clearly higher than amongst the general population; furthermore, there is a higher number of adverse effects in the group receiving no treatment. Although in the series of cases in hand, therapy using LMWH and/or ATIII has been demonstrated as an effective and safe strategy, these patients must be subject to strict monitoring, as complications can occur throughout the pregnancy and post-partum period, particularly in the case of inadequate thromboprophylaxis. Further research is needed to establish and standardise action protocols.

Session topic: E-poster
Keyword(s): Antithrombin, Pregnancy
Abstract: E1564
Type: Eposter Presentation
Background
During pregnancy, the risk of suffering thromboembolic disease increases fivefold. The most significant risk factors are a prior history of thrombosis followed by the presence of thrombophilia, with ATIII deficiency associated to a greater risk of thrombosis and complications during pregnancy (including miscarriage, intrauterine growth restriction, fetal death, placental abruption, pre-eclampsia and HELLP syndrome). The ideal treatment for such women remains undefined, given that limited clinical data is available and most cases are reported on an individual basis or on a small scale. It is widely accepted that most of these patients should receive thromboembolic prophylaxis, although the dosage, duration and type of anticoagulant remains a contentious issue.
Aims
Evaluate the impact of antithrombin deficiency and the different treatments in pregnant women.
Methods
Retrospective 30-year study of pregnant women with ATIII deficiency. We analysed a total of 57 pregnancies in 21 women diagnosed with ATIII deficiency (10 due to family history, 2 due to a child having shown the deficiency and the remainder due to personal history). The average age was 29 (19-41). 4 pregnant women displayed cardiovascular risk factors (1 high blood pressure, 1 smoker, 1 dyslipidemia and the other a smoker plus sufferer of dyslipidemia) and 8 had additional thrombophilia (6 with heterozygous Factor V Leiden, one protein S deficiency and one combined homozygous factor XII deficiency and heterozygous Factor V Leiden).
Results
Of the 57 pregnant women, 2 of whom were carrying twins, 38 received no treatment as the antithrombin III deficiency was diagnosed at a later date. From this group, 22 resulted in live births, 12 miscarried, 6 suffered an intra-uterine stillbirth and 2 experienced intrauterine growth retardation. In terms of maternal complications that went untreated, 2 women developed pre-eclampsia and there were 5 thrombotic events (2 pulmonary thromboembolisms, one of which with sinus-related thrombosis and 4 deep vein thromboses). As part of 6 pregnancies, patients received Acenocumarol due to a history of thromboses.19 pregnant women received treatment: 15 low molecular weight heparin (LMWH) in therapeutic doses subject to Anti Xa control (6 received Acenocumarol previously due to a history of thromboses), 1 LMWH in intermediate doses and 3 in prophylactic doses. At the same time, ATIII 50UI/kg/72 hours was administered to 4 patients and 2 took Acenocumarol from week 12. During the peripartum period, two received LMWH, another ATIII and 13 ATIII linked to LMWH in prophylactic doses. Post-partum, 9 patients received Acenocumarol (6 having received bridging treatment via ATIII and LMWH and 3 just via LMWH) and 10 LMWH (5 of which with ATIII).From amongst the 19 pregnancies as part of which treatment was administered, 17 resulted in live births. Complications recorded included one pre-eclampsia, one miscarriage and one maternal death due to severe sinus thrombosis, all three of which occurred in patients receiving a prophylactic dose of Heparin. The only adverse effect of the treatment involved bleeding from a caesarean wound in one of the patients receiving ATIII and LMWH. 10 received an epidural with no adverse effects.
Conclusion
In our experience, the rate of maternal-fetal complications in patients with an ATIII deficiency is clearly higher than amongst the general population; furthermore, there is a higher number of adverse effects in the group receiving no treatment. Although in the series of cases in hand, therapy using LMWH and/or ATIII has been demonstrated as an effective and safe strategy, these patients must be subject to strict monitoring, as complications can occur throughout the pregnancy and post-partum period, particularly in the case of inadequate thromboprophylaxis. Further research is needed to establish and standardise action protocols.

Session topic: E-poster
Keyword(s): Antithrombin, Pregnancy
Type: Eposter Presentation
Background
During pregnancy, the risk of suffering thromboembolic disease increases fivefold. The most significant risk factors are a prior history of thrombosis followed by the presence of thrombophilia, with ATIII deficiency associated to a greater risk of thrombosis and complications during pregnancy (including miscarriage, intrauterine growth restriction, fetal death, placental abruption, pre-eclampsia and HELLP syndrome). The ideal treatment for such women remains undefined, given that limited clinical data is available and most cases are reported on an individual basis or on a small scale. It is widely accepted that most of these patients should receive thromboembolic prophylaxis, although the dosage, duration and type of anticoagulant remains a contentious issue.
Aims
Evaluate the impact of antithrombin deficiency and the different treatments in pregnant women.
Methods
Retrospective 30-year study of pregnant women with ATIII deficiency. We analysed a total of 57 pregnancies in 21 women diagnosed with ATIII deficiency (10 due to family history, 2 due to a child having shown the deficiency and the remainder due to personal history). The average age was 29 (19-41). 4 pregnant women displayed cardiovascular risk factors (1 high blood pressure, 1 smoker, 1 dyslipidemia and the other a smoker plus sufferer of dyslipidemia) and 8 had additional thrombophilia (6 with heterozygous Factor V Leiden, one protein S deficiency and one combined homozygous factor XII deficiency and heterozygous Factor V Leiden).
Results
Of the 57 pregnant women, 2 of whom were carrying twins, 38 received no treatment as the antithrombin III deficiency was diagnosed at a later date. From this group, 22 resulted in live births, 12 miscarried, 6 suffered an intra-uterine stillbirth and 2 experienced intrauterine growth retardation. In terms of maternal complications that went untreated, 2 women developed pre-eclampsia and there were 5 thrombotic events (2 pulmonary thromboembolisms, one of which with sinus-related thrombosis and 4 deep vein thromboses). As part of 6 pregnancies, patients received Acenocumarol due to a history of thromboses.19 pregnant women received treatment: 15 low molecular weight heparin (LMWH) in therapeutic doses subject to Anti Xa control (6 received Acenocumarol previously due to a history of thromboses), 1 LMWH in intermediate doses and 3 in prophylactic doses. At the same time, ATIII 50UI/kg/72 hours was administered to 4 patients and 2 took Acenocumarol from week 12. During the peripartum period, two received LMWH, another ATIII and 13 ATIII linked to LMWH in prophylactic doses. Post-partum, 9 patients received Acenocumarol (6 having received bridging treatment via ATIII and LMWH and 3 just via LMWH) and 10 LMWH (5 of which with ATIII).From amongst the 19 pregnancies as part of which treatment was administered, 17 resulted in live births. Complications recorded included one pre-eclampsia, one miscarriage and one maternal death due to severe sinus thrombosis, all three of which occurred in patients receiving a prophylactic dose of Heparin. The only adverse effect of the treatment involved bleeding from a caesarean wound in one of the patients receiving ATIII and LMWH. 10 received an epidural with no adverse effects.
Conclusion
In our experience, the rate of maternal-fetal complications in patients with an ATIII deficiency is clearly higher than amongst the general population; furthermore, there is a higher number of adverse effects in the group receiving no treatment. Although in the series of cases in hand, therapy using LMWH and/or ATIII has been demonstrated as an effective and safe strategy, these patients must be subject to strict monitoring, as complications can occur throughout the pregnancy and post-partum period, particularly in the case of inadequate thromboprophylaxis. Further research is needed to establish and standardise action protocols.

Session topic: E-poster
Keyword(s): Antithrombin, Pregnancy
{{ help_message }}
{{filter}}


