A COMPARISON OF ORIGINATOR G-CSFS VERSUS BIOSIMILAR G-CSFS AFTER AUTOGRAFTING IN HEMATOLOGICAL MALIGNANCIES.
(Abstract release date: 05/19/16)
EHA Library. Brunello L. 06/09/16; 133097; E1548

Dr. Lucia Brunello
Contributions
Contributions
Abstract
Abstract: E1548
Type: Eposter Presentation
Background
Autografting is widely used for the treatment of hematological malignancies, especially for lymphoproliferative diseases. In recent years, biosimilar G-CSFs (BioG-CSFs) have gradually been introduced into clinical practice to mobilize peripheral blood hematopoietic stem cells (CD34+ cells) and reduce the duration of neutropenia.
Aims
This study was designed to assess, in the setting of “real life” clinical practice, the role of BioG-CSFs and to compare them with a historical patient cohort treated with “originator” G-CSFs after autografting. The study was prompted by the growing propensity for the use of biosimilar growth factors to reduce overall costs and by the imminent implementation of other novel biosimilar molecules.
Methods
The study period was 2006-2015 during which standard policies for autografting did not change. Primary endpoints were post transplant engraftment kinetics, transfusion requirements and duration of hospitalization. Secondary objective was one-year overall survival. Day of neutrophil engraftment was defined as the first of 3 consecutive days of absolute neutrophil count ≥500/ul whereas day of platelet engraftment was defined as the first of 7 consecutive days of platelets ≥20.000/ul unsupported by transfusion.
Results
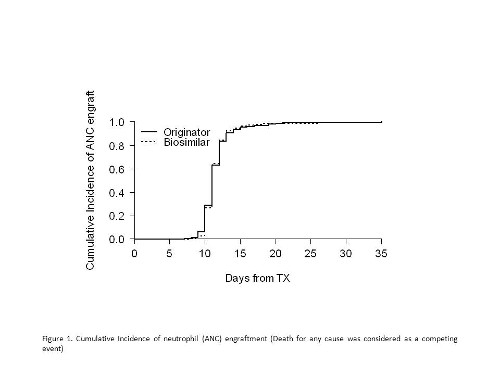
276 patients were studied for a total of 354 transplants. Underlying diseases included multiple myeloma (n=204), non-Hodgkin lymphoma (n=56), Hodgkin lymphoma (n=9), chronic lymphocytic leukemia (n=2), acute myeloid leukemia (n=4) and histiocytic sarcoma (n=1). Seventy-eight multiple myeloma patients received double autologous transplant. Conditionings were as follows: melphalan 200 mg/m2 for myeloma; busulfan/cyclophosphamide for acute myeloid leukemia; BEAM or FEAM-like for lymphoproliferative disorders. Overall,148 and 206 patients received originator G-CSFs and BioG-CSFs respectively. Infused CD34+ cells were 4.99 (IQR 3.77-5.05) x106/kg/body weight and 4.17 (IQR 3.66-5.00) x106/kg/body weight in the originator G-CSFs and BioG-CSFs cohorts. Cumulative incidences of neutrophil recovery at day +15 and +25 were 95.3% (95% CI 91.7% - 98.8%) and 99.3% (95% CI 97.6% - 100%) and 96.1% (95% CI 93.4% - 98.8%) and 98.5% (95% CI 96.8% - 100%) in the originator G-CSFs and BioG-CSFs groups respectively (p=0.980). These data were confirmed by Propensity Score adjustment, HR 1.03 (95% CI 0.87-1.2), p= 0.763. Cumulative incidence of platelet recovery at day +30 was 98.6% (95% CI 96.5% - 100%) and 96.1% (95% CI 93.4% - 98.8%) in the originator G-CSFs and BioG-CSFs cohorts (p<0.001). Of note, no differences between cohorts were found in a) median duration of neutropenia (median: 7 and 6 days, p=0.180) b) platelet (median 1 pool/patient in both, p=0.441) and red blood cell (median: 0/patient in both, p=0.703) transfusion requirements, hospital stay (median: 20 days in both, p=0.270). Interestingly, engraftment kinetics after the second transplant were faster for both neutrophil and platelet recoveries (HR 1.34 (95% CI 1.1-1.62), p=0.003 and HR 1.30 (95% CI 1.01-1.67), p=0.038, respectively, by multivariate models). No severe adverse reactions attributable to growth factors were documented. Finally, one-year overall survival was comparable between cohorts (p=0.901).
Conclusion
In this sizable study, BioG-CSFs were as effective as originator G-CSFs. Moreover, their extensive use led to a significant cost containment.
Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Filgrastim, Granulocyte colony-stimulating factor (G-CSF)
Type: Eposter Presentation
Background
Autografting is widely used for the treatment of hematological malignancies, especially for lymphoproliferative diseases. In recent years, biosimilar G-CSFs (BioG-CSFs) have gradually been introduced into clinical practice to mobilize peripheral blood hematopoietic stem cells (CD34+ cells) and reduce the duration of neutropenia.
Aims
This study was designed to assess, in the setting of “real life” clinical practice, the role of BioG-CSFs and to compare them with a historical patient cohort treated with “originator” G-CSFs after autografting. The study was prompted by the growing propensity for the use of biosimilar growth factors to reduce overall costs and by the imminent implementation of other novel biosimilar molecules.
Methods
The study period was 2006-2015 during which standard policies for autografting did not change. Primary endpoints were post transplant engraftment kinetics, transfusion requirements and duration of hospitalization. Secondary objective was one-year overall survival. Day of neutrophil engraftment was defined as the first of 3 consecutive days of absolute neutrophil count ≥500/ul whereas day of platelet engraftment was defined as the first of 7 consecutive days of platelets ≥20.000/ul unsupported by transfusion.
Results
276 patients were studied for a total of 354 transplants. Underlying diseases included multiple myeloma (n=204), non-Hodgkin lymphoma (n=56), Hodgkin lymphoma (n=9), chronic lymphocytic leukemia (n=2), acute myeloid leukemia (n=4) and histiocytic sarcoma (n=1). Seventy-eight multiple myeloma patients received double autologous transplant. Conditionings were as follows: melphalan 200 mg/m2 for myeloma; busulfan/cyclophosphamide for acute myeloid leukemia; BEAM or FEAM-like for lymphoproliferative disorders. Overall,148 and 206 patients received originator G-CSFs and BioG-CSFs respectively. Infused CD34+ cells were 4.99 (IQR 3.77-5.05) x106/kg/body weight and 4.17 (IQR 3.66-5.00) x106/kg/body weight in the originator G-CSFs and BioG-CSFs cohorts. Cumulative incidences of neutrophil recovery at day +15 and +25 were 95.3% (95% CI 91.7% - 98.8%) and 99.3% (95% CI 97.6% - 100%) and 96.1% (95% CI 93.4% - 98.8%) and 98.5% (95% CI 96.8% - 100%) in the originator G-CSFs and BioG-CSFs groups respectively (p=0.980). These data were confirmed by Propensity Score adjustment, HR 1.03 (95% CI 0.87-1.2), p= 0.763. Cumulative incidence of platelet recovery at day +30 was 98.6% (95% CI 96.5% - 100%) and 96.1% (95% CI 93.4% - 98.8%) in the originator G-CSFs and BioG-CSFs cohorts (p<0.001). Of note, no differences between cohorts were found in a) median duration of neutropenia (median: 7 and 6 days, p=0.180) b) platelet (median 1 pool/patient in both, p=0.441) and red blood cell (median: 0/patient in both, p=0.703) transfusion requirements, hospital stay (median: 20 days in both, p=0.270). Interestingly, engraftment kinetics after the second transplant were faster for both neutrophil and platelet recoveries (HR 1.34 (95% CI 1.1-1.62), p=0.003 and HR 1.30 (95% CI 1.01-1.67), p=0.038, respectively, by multivariate models). No severe adverse reactions attributable to growth factors were documented. Finally, one-year overall survival was comparable between cohorts (p=0.901).
Conclusion
In this sizable study, BioG-CSFs were as effective as originator G-CSFs. Moreover, their extensive use led to a significant cost containment.

Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Filgrastim, Granulocyte colony-stimulating factor (G-CSF)
Abstract: E1548
Type: Eposter Presentation
Background
Autografting is widely used for the treatment of hematological malignancies, especially for lymphoproliferative diseases. In recent years, biosimilar G-CSFs (BioG-CSFs) have gradually been introduced into clinical practice to mobilize peripheral blood hematopoietic stem cells (CD34+ cells) and reduce the duration of neutropenia.
Aims
This study was designed to assess, in the setting of “real life” clinical practice, the role of BioG-CSFs and to compare them with a historical patient cohort treated with “originator” G-CSFs after autografting. The study was prompted by the growing propensity for the use of biosimilar growth factors to reduce overall costs and by the imminent implementation of other novel biosimilar molecules.
Methods
The study period was 2006-2015 during which standard policies for autografting did not change. Primary endpoints were post transplant engraftment kinetics, transfusion requirements and duration of hospitalization. Secondary objective was one-year overall survival. Day of neutrophil engraftment was defined as the first of 3 consecutive days of absolute neutrophil count ≥500/ul whereas day of platelet engraftment was defined as the first of 7 consecutive days of platelets ≥20.000/ul unsupported by transfusion.
Results
276 patients were studied for a total of 354 transplants. Underlying diseases included multiple myeloma (n=204), non-Hodgkin lymphoma (n=56), Hodgkin lymphoma (n=9), chronic lymphocytic leukemia (n=2), acute myeloid leukemia (n=4) and histiocytic sarcoma (n=1). Seventy-eight multiple myeloma patients received double autologous transplant. Conditionings were as follows: melphalan 200 mg/m2 for myeloma; busulfan/cyclophosphamide for acute myeloid leukemia; BEAM or FEAM-like for lymphoproliferative disorders. Overall,148 and 206 patients received originator G-CSFs and BioG-CSFs respectively. Infused CD34+ cells were 4.99 (IQR 3.77-5.05) x106/kg/body weight and 4.17 (IQR 3.66-5.00) x106/kg/body weight in the originator G-CSFs and BioG-CSFs cohorts. Cumulative incidences of neutrophil recovery at day +15 and +25 were 95.3% (95% CI 91.7% - 98.8%) and 99.3% (95% CI 97.6% - 100%) and 96.1% (95% CI 93.4% - 98.8%) and 98.5% (95% CI 96.8% - 100%) in the originator G-CSFs and BioG-CSFs groups respectively (p=0.980). These data were confirmed by Propensity Score adjustment, HR 1.03 (95% CI 0.87-1.2), p= 0.763. Cumulative incidence of platelet recovery at day +30 was 98.6% (95% CI 96.5% - 100%) and 96.1% (95% CI 93.4% - 98.8%) in the originator G-CSFs and BioG-CSFs cohorts (p<0.001). Of note, no differences between cohorts were found in a) median duration of neutropenia (median: 7 and 6 days, p=0.180) b) platelet (median 1 pool/patient in both, p=0.441) and red blood cell (median: 0/patient in both, p=0.703) transfusion requirements, hospital stay (median: 20 days in both, p=0.270). Interestingly, engraftment kinetics after the second transplant were faster for both neutrophil and platelet recoveries (HR 1.34 (95% CI 1.1-1.62), p=0.003 and HR 1.30 (95% CI 1.01-1.67), p=0.038, respectively, by multivariate models). No severe adverse reactions attributable to growth factors were documented. Finally, one-year overall survival was comparable between cohorts (p=0.901).
Conclusion
In this sizable study, BioG-CSFs were as effective as originator G-CSFs. Moreover, their extensive use led to a significant cost containment.

Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Filgrastim, Granulocyte colony-stimulating factor (G-CSF)
Type: Eposter Presentation
Background
Autografting is widely used for the treatment of hematological malignancies, especially for lymphoproliferative diseases. In recent years, biosimilar G-CSFs (BioG-CSFs) have gradually been introduced into clinical practice to mobilize peripheral blood hematopoietic stem cells (CD34+ cells) and reduce the duration of neutropenia.
Aims
This study was designed to assess, in the setting of “real life” clinical practice, the role of BioG-CSFs and to compare them with a historical patient cohort treated with “originator” G-CSFs after autografting. The study was prompted by the growing propensity for the use of biosimilar growth factors to reduce overall costs and by the imminent implementation of other novel biosimilar molecules.
Methods
The study period was 2006-2015 during which standard policies for autografting did not change. Primary endpoints were post transplant engraftment kinetics, transfusion requirements and duration of hospitalization. Secondary objective was one-year overall survival. Day of neutrophil engraftment was defined as the first of 3 consecutive days of absolute neutrophil count ≥500/ul whereas day of platelet engraftment was defined as the first of 7 consecutive days of platelets ≥20.000/ul unsupported by transfusion.
Results
276 patients were studied for a total of 354 transplants. Underlying diseases included multiple myeloma (n=204), non-Hodgkin lymphoma (n=56), Hodgkin lymphoma (n=9), chronic lymphocytic leukemia (n=2), acute myeloid leukemia (n=4) and histiocytic sarcoma (n=1). Seventy-eight multiple myeloma patients received double autologous transplant. Conditionings were as follows: melphalan 200 mg/m2 for myeloma; busulfan/cyclophosphamide for acute myeloid leukemia; BEAM or FEAM-like for lymphoproliferative disorders. Overall,148 and 206 patients received originator G-CSFs and BioG-CSFs respectively. Infused CD34+ cells were 4.99 (IQR 3.77-5.05) x106/kg/body weight and 4.17 (IQR 3.66-5.00) x106/kg/body weight in the originator G-CSFs and BioG-CSFs cohorts. Cumulative incidences of neutrophil recovery at day +15 and +25 were 95.3% (95% CI 91.7% - 98.8%) and 99.3% (95% CI 97.6% - 100%) and 96.1% (95% CI 93.4% - 98.8%) and 98.5% (95% CI 96.8% - 100%) in the originator G-CSFs and BioG-CSFs groups respectively (p=0.980). These data were confirmed by Propensity Score adjustment, HR 1.03 (95% CI 0.87-1.2), p= 0.763. Cumulative incidence of platelet recovery at day +30 was 98.6% (95% CI 96.5% - 100%) and 96.1% (95% CI 93.4% - 98.8%) in the originator G-CSFs and BioG-CSFs cohorts (p<0.001). Of note, no differences between cohorts were found in a) median duration of neutropenia (median: 7 and 6 days, p=0.180) b) platelet (median 1 pool/patient in both, p=0.441) and red blood cell (median: 0/patient in both, p=0.703) transfusion requirements, hospital stay (median: 20 days in both, p=0.270). Interestingly, engraftment kinetics after the second transplant were faster for both neutrophil and platelet recoveries (HR 1.34 (95% CI 1.1-1.62), p=0.003 and HR 1.30 (95% CI 1.01-1.67), p=0.038, respectively, by multivariate models). No severe adverse reactions attributable to growth factors were documented. Finally, one-year overall survival was comparable between cohorts (p=0.901).
Conclusion
In this sizable study, BioG-CSFs were as effective as originator G-CSFs. Moreover, their extensive use led to a significant cost containment.

Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Filgrastim, Granulocyte colony-stimulating factor (G-CSF)
{{ help_message }}
{{filter}}


