A PHASE 2, MULTICENTER, SINGLE-ARM STUDY TO EVALUATE SAFETY AND EFFICACY OF DEFERASIROX AFTER HEMATOPOIETIC STEM CELL TRANSPLANTATION IN CHILDREN WITH BETA-THALASSEMIA MAJOR
(Abstract release date: 05/19/16)
EHA Library. Akif Yesilipek M. 06/09/16; 133066; E1517

Dr. Mehmet Akif Yesilipek
Contributions
Contributions
Abstract
Abstract: E1517
Type: Eposter Presentation
Background
Hematopoietic stem cell transplantation (HSCT) is being increasingly used as curative therapy for severe disorders of the hematopoietic system such as thalassemia. The use of therapeutic phlebotomy post-HSCT is often difficult to perform in younger or anemic children. Deferiprone is shown to be related with bone marrow suppression and compliance to deferoxamine is low. Studies that evaluate the safety of deferasirox (DFX) in this setting, are limited.
Aims
The aim of this study is to evaluate safety and efficacy of DFX in Thalassemia Major patients in a Post Transplant setting.
Methods
This was a prospective, phase 2, multicenter, single-arm study to evaluate efficacy and safety of DFX in beta-thalassemia major (TM) patients who have undergone HSCT. The primary objective was to evaluate if DFX could provide safe chelation in patients with transfusional iron overload within a time period of 6 months to 2 years after HSCT. Transfusion independent patients aged ≥2 to <18 years old who had undergone HSCT with a washout period of at least 3 months after immunosuppressive therapies and had iron overload at screening defined by serum ferritin (SF) of >1000 μg/L or cardiac MRI T2* <20 ms or liver iron concentration (LIC, by MRI R2) of ≥5 mg/g were included in the study. Patients received DFX at an initial dose of 10 mg/kg/day with up titration every 3 months by 5 mg/kg/day per investigator judgment to the intended 20 mg/kg/day dose. Therapy continued for 52 weeks or until SF reached below 500 μg/L.
Results
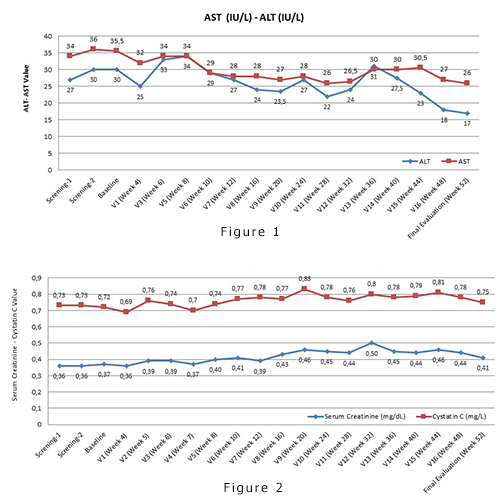
Data from 27 patients enrolled (median (range) age 9.07 years (3-16), 70,4% males) were included in this analysis. One patient discontinued on week 14 due to withdrawal of consent. 20 patients were able to achieve the intended dose of 20 mg/kg/day.SafetyFrom a total of 140 Adverse Events (AEs), 10 (7.1%) AEs in 4 patients were suspected to be related to study drug. The majority of AEs were of Grade I (n=81, 57.9%) or II (n=48, 34.3%). A total number of 6 (4.7%) SAE’s were reported. 8 (5.7%) AEs resulted in study drug interruption or dose adjustment, 1 of which was suspected to be related to the study drug (ALT increase). On 51 (36.4%)AE’s, no action was taken. Median ALT level decreased from 27 IU/L (range: 10-119) at baseline to 17 IU/L (range: 9-205) at week 52. Median AST level decreased from 35,5 IU/L (range: 17-66.5) to 26 IU/L (range:18-78) at week 52 (Figure 1). The median SCr was similar at baseline (0.4 mg/dL range: 0.2-0.7) and week 52 (0.4 mg/dL range: 0.2-1) Median cystatin C was similar at baseline (0.7 mg/mL range: 0.5-1) and week 52 (0.7 mg/mL range: 0.5-1) (Figure 2). No dose adjustments were required due to nephrotoxicity. Increased proteinuria was described in 9 (33.3%) patients, irrespective of dose at 52 weeks. No patient with proteinuria required dose adjustments.EfficacySF significantly and consistently decreased through 52 weeks from a median of 1718 μg/L (range: 873.7-2919) to 845.3 μg/L (range: 146.2-2740), p<0.001. At week 52, 9 (33.3%) patients reached SF <500 μg/L, p<0.001. LIC also significantly decreased from a median of 8.60 mg/g (range: 2.8-43) to 4.1 mg/g (range 0.9-12.5), p<0.001. Cardiac T2* increased from a median of 25.95 ms (range: 4.5-51) to 28 ms (18.5-44), p=0.520.
Conclusion
Our findings suggest that DFX dose escalation up to 15-20 mg/kg/day is safe for TM patients following successful HSCT regarding nephrotoxicity and hepatotoxicity. 15-20 mg/kg/day deferasirox was also efficacious and this was evident through significant reductions of systemic and hepatic iron overload.
Session topic: E-poster
Keyword(s): Chelation, Deferasirox, HSCT, Thalassemia
Type: Eposter Presentation
Background
Hematopoietic stem cell transplantation (HSCT) is being increasingly used as curative therapy for severe disorders of the hematopoietic system such as thalassemia. The use of therapeutic phlebotomy post-HSCT is often difficult to perform in younger or anemic children. Deferiprone is shown to be related with bone marrow suppression and compliance to deferoxamine is low. Studies that evaluate the safety of deferasirox (DFX) in this setting, are limited.
Aims
The aim of this study is to evaluate safety and efficacy of DFX in Thalassemia Major patients in a Post Transplant setting.
Methods
This was a prospective, phase 2, multicenter, single-arm study to evaluate efficacy and safety of DFX in beta-thalassemia major (TM) patients who have undergone HSCT. The primary objective was to evaluate if DFX could provide safe chelation in patients with transfusional iron overload within a time period of 6 months to 2 years after HSCT. Transfusion independent patients aged ≥2 to <18 years old who had undergone HSCT with a washout period of at least 3 months after immunosuppressive therapies and had iron overload at screening defined by serum ferritin (SF) of >1000 μg/L or cardiac MRI T2* <20 ms or liver iron concentration (LIC, by MRI R2) of ≥5 mg/g were included in the study. Patients received DFX at an initial dose of 10 mg/kg/day with up titration every 3 months by 5 mg/kg/day per investigator judgment to the intended 20 mg/kg/day dose. Therapy continued for 52 weeks or until SF reached below 500 μg/L.
Results
Data from 27 patients enrolled (median (range) age 9.07 years (3-16), 70,4% males) were included in this analysis. One patient discontinued on week 14 due to withdrawal of consent. 20 patients were able to achieve the intended dose of 20 mg/kg/day.SafetyFrom a total of 140 Adverse Events (AEs), 10 (7.1%) AEs in 4 patients were suspected to be related to study drug. The majority of AEs were of Grade I (n=81, 57.9%) or II (n=48, 34.3%). A total number of 6 (4.7%) SAE’s were reported. 8 (5.7%) AEs resulted in study drug interruption or dose adjustment, 1 of which was suspected to be related to the study drug (ALT increase). On 51 (36.4%)AE’s, no action was taken. Median ALT level decreased from 27 IU/L (range: 10-119) at baseline to 17 IU/L (range: 9-205) at week 52. Median AST level decreased from 35,5 IU/L (range: 17-66.5) to 26 IU/L (range:18-78) at week 52 (Figure 1). The median SCr was similar at baseline (0.4 mg/dL range: 0.2-0.7) and week 52 (0.4 mg/dL range: 0.2-1) Median cystatin C was similar at baseline (0.7 mg/mL range: 0.5-1) and week 52 (0.7 mg/mL range: 0.5-1) (Figure 2). No dose adjustments were required due to nephrotoxicity. Increased proteinuria was described in 9 (33.3%) patients, irrespective of dose at 52 weeks. No patient with proteinuria required dose adjustments.EfficacySF significantly and consistently decreased through 52 weeks from a median of 1718 μg/L (range: 873.7-2919) to 845.3 μg/L (range: 146.2-2740), p<0.001. At week 52, 9 (33.3%) patients reached SF <500 μg/L, p<0.001. LIC also significantly decreased from a median of 8.60 mg/g (range: 2.8-43) to 4.1 mg/g (range 0.9-12.5), p<0.001. Cardiac T2* increased from a median of 25.95 ms (range: 4.5-51) to 28 ms (18.5-44), p=0.520.
Conclusion
Our findings suggest that DFX dose escalation up to 15-20 mg/kg/day is safe for TM patients following successful HSCT regarding nephrotoxicity and hepatotoxicity. 15-20 mg/kg/day deferasirox was also efficacious and this was evident through significant reductions of systemic and hepatic iron overload.

Session topic: E-poster
Keyword(s): Chelation, Deferasirox, HSCT, Thalassemia
Abstract: E1517
Type: Eposter Presentation
Background
Hematopoietic stem cell transplantation (HSCT) is being increasingly used as curative therapy for severe disorders of the hematopoietic system such as thalassemia. The use of therapeutic phlebotomy post-HSCT is often difficult to perform in younger or anemic children. Deferiprone is shown to be related with bone marrow suppression and compliance to deferoxamine is low. Studies that evaluate the safety of deferasirox (DFX) in this setting, are limited.
Aims
The aim of this study is to evaluate safety and efficacy of DFX in Thalassemia Major patients in a Post Transplant setting.
Methods
This was a prospective, phase 2, multicenter, single-arm study to evaluate efficacy and safety of DFX in beta-thalassemia major (TM) patients who have undergone HSCT. The primary objective was to evaluate if DFX could provide safe chelation in patients with transfusional iron overload within a time period of 6 months to 2 years after HSCT. Transfusion independent patients aged ≥2 to <18 years old who had undergone HSCT with a washout period of at least 3 months after immunosuppressive therapies and had iron overload at screening defined by serum ferritin (SF) of >1000 μg/L or cardiac MRI T2* <20 ms or liver iron concentration (LIC, by MRI R2) of ≥5 mg/g were included in the study. Patients received DFX at an initial dose of 10 mg/kg/day with up titration every 3 months by 5 mg/kg/day per investigator judgment to the intended 20 mg/kg/day dose. Therapy continued for 52 weeks or until SF reached below 500 μg/L.
Results
Data from 27 patients enrolled (median (range) age 9.07 years (3-16), 70,4% males) were included in this analysis. One patient discontinued on week 14 due to withdrawal of consent. 20 patients were able to achieve the intended dose of 20 mg/kg/day.SafetyFrom a total of 140 Adverse Events (AEs), 10 (7.1%) AEs in 4 patients were suspected to be related to study drug. The majority of AEs were of Grade I (n=81, 57.9%) or II (n=48, 34.3%). A total number of 6 (4.7%) SAE’s were reported. 8 (5.7%) AEs resulted in study drug interruption or dose adjustment, 1 of which was suspected to be related to the study drug (ALT increase). On 51 (36.4%)AE’s, no action was taken. Median ALT level decreased from 27 IU/L (range: 10-119) at baseline to 17 IU/L (range: 9-205) at week 52. Median AST level decreased from 35,5 IU/L (range: 17-66.5) to 26 IU/L (range:18-78) at week 52 (Figure 1). The median SCr was similar at baseline (0.4 mg/dL range: 0.2-0.7) and week 52 (0.4 mg/dL range: 0.2-1) Median cystatin C was similar at baseline (0.7 mg/mL range: 0.5-1) and week 52 (0.7 mg/mL range: 0.5-1) (Figure 2). No dose adjustments were required due to nephrotoxicity. Increased proteinuria was described in 9 (33.3%) patients, irrespective of dose at 52 weeks. No patient with proteinuria required dose adjustments.EfficacySF significantly and consistently decreased through 52 weeks from a median of 1718 μg/L (range: 873.7-2919) to 845.3 μg/L (range: 146.2-2740), p<0.001. At week 52, 9 (33.3%) patients reached SF <500 μg/L, p<0.001. LIC also significantly decreased from a median of 8.60 mg/g (range: 2.8-43) to 4.1 mg/g (range 0.9-12.5), p<0.001. Cardiac T2* increased from a median of 25.95 ms (range: 4.5-51) to 28 ms (18.5-44), p=0.520.
Conclusion
Our findings suggest that DFX dose escalation up to 15-20 mg/kg/day is safe for TM patients following successful HSCT regarding nephrotoxicity and hepatotoxicity. 15-20 mg/kg/day deferasirox was also efficacious and this was evident through significant reductions of systemic and hepatic iron overload.

Session topic: E-poster
Keyword(s): Chelation, Deferasirox, HSCT, Thalassemia
Type: Eposter Presentation
Background
Hematopoietic stem cell transplantation (HSCT) is being increasingly used as curative therapy for severe disorders of the hematopoietic system such as thalassemia. The use of therapeutic phlebotomy post-HSCT is often difficult to perform in younger or anemic children. Deferiprone is shown to be related with bone marrow suppression and compliance to deferoxamine is low. Studies that evaluate the safety of deferasirox (DFX) in this setting, are limited.
Aims
The aim of this study is to evaluate safety and efficacy of DFX in Thalassemia Major patients in a Post Transplant setting.
Methods
This was a prospective, phase 2, multicenter, single-arm study to evaluate efficacy and safety of DFX in beta-thalassemia major (TM) patients who have undergone HSCT. The primary objective was to evaluate if DFX could provide safe chelation in patients with transfusional iron overload within a time period of 6 months to 2 years after HSCT. Transfusion independent patients aged ≥2 to <18 years old who had undergone HSCT with a washout period of at least 3 months after immunosuppressive therapies and had iron overload at screening defined by serum ferritin (SF) of >1000 μg/L or cardiac MRI T2* <20 ms or liver iron concentration (LIC, by MRI R2) of ≥5 mg/g were included in the study. Patients received DFX at an initial dose of 10 mg/kg/day with up titration every 3 months by 5 mg/kg/day per investigator judgment to the intended 20 mg/kg/day dose. Therapy continued for 52 weeks or until SF reached below 500 μg/L.
Results
Data from 27 patients enrolled (median (range) age 9.07 years (3-16), 70,4% males) were included in this analysis. One patient discontinued on week 14 due to withdrawal of consent. 20 patients were able to achieve the intended dose of 20 mg/kg/day.SafetyFrom a total of 140 Adverse Events (AEs), 10 (7.1%) AEs in 4 patients were suspected to be related to study drug. The majority of AEs were of Grade I (n=81, 57.9%) or II (n=48, 34.3%). A total number of 6 (4.7%) SAE’s were reported. 8 (5.7%) AEs resulted in study drug interruption or dose adjustment, 1 of which was suspected to be related to the study drug (ALT increase). On 51 (36.4%)AE’s, no action was taken. Median ALT level decreased from 27 IU/L (range: 10-119) at baseline to 17 IU/L (range: 9-205) at week 52. Median AST level decreased from 35,5 IU/L (range: 17-66.5) to 26 IU/L (range:18-78) at week 52 (Figure 1). The median SCr was similar at baseline (0.4 mg/dL range: 0.2-0.7) and week 52 (0.4 mg/dL range: 0.2-1) Median cystatin C was similar at baseline (0.7 mg/mL range: 0.5-1) and week 52 (0.7 mg/mL range: 0.5-1) (Figure 2). No dose adjustments were required due to nephrotoxicity. Increased proteinuria was described in 9 (33.3%) patients, irrespective of dose at 52 weeks. No patient with proteinuria required dose adjustments.EfficacySF significantly and consistently decreased through 52 weeks from a median of 1718 μg/L (range: 873.7-2919) to 845.3 μg/L (range: 146.2-2740), p<0.001. At week 52, 9 (33.3%) patients reached SF <500 μg/L, p<0.001. LIC also significantly decreased from a median of 8.60 mg/g (range: 2.8-43) to 4.1 mg/g (range 0.9-12.5), p<0.001. Cardiac T2* increased from a median of 25.95 ms (range: 4.5-51) to 28 ms (18.5-44), p=0.520.
Conclusion
Our findings suggest that DFX dose escalation up to 15-20 mg/kg/day is safe for TM patients following successful HSCT regarding nephrotoxicity and hepatotoxicity. 15-20 mg/kg/day deferasirox was also efficacious and this was evident through significant reductions of systemic and hepatic iron overload.

Session topic: E-poster
Keyword(s): Chelation, Deferasirox, HSCT, Thalassemia
{{ help_message }}
{{filter}}


