“REAL-LIFE” REPORT ON THE MANAGEMENT OF CHRONIC GRAFT-VERSUS-HOST DISEASE: A SURVEY CONDUCTED ON BEHALF OF GITMO (GRUPPO ITALIANO TRAPIANTO MIDOLLO OSSEO)
(Abstract release date: 05/19/16)
EHA Library. Olivieri A. 06/09/16; 133065; E1516
Disclosure(s): nothing to declare

Attilio Olivieri
Contributions
Contributions
Abstract
Abstract: E1516
Type: Eposter Presentation
Background
Diagnosing, scoring and treating chronic graft-versus-host disease (cGVHD) is challenging because of polymorphic manifestations and lack of biomarkers for the diagnosis and assessment of disease activity. Several guidelines have been published, however the clinical practice remains demanding.
Aims
With this study we investigated the 'real life' management of cGVHD in 32 Italian Center members of GITMO (Gruppo Italiano Trapianto Midollo Osseo).
Methods
A detailed survey with 41 multiple-choice questions about the use of guidelines, and the first and further lines of treatment and management of cGVHD has been proposed to all adult and pediatric GITMO Centers (N=60)
Results
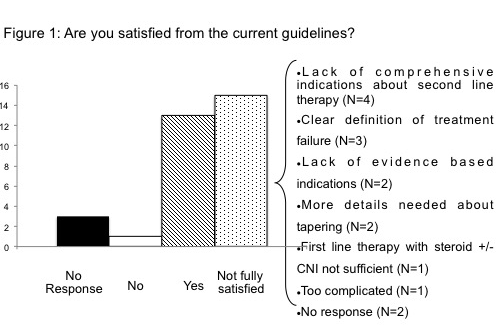
Thirty-two Centers replied, and according to the survey's results, 29 Centers referred to published guidelines for cGVHD management (mainly those proposed by National Institute of Health, NIH), however only 13 find them fully adequate. As shown in Figure 1, the main reasons for complain were related to second line treatment. According to NIH definition, most of the Centers (N=25) started treatment when severe cGVHD occurred, whereas 4 Centers also considered bad prognostic features regardless the grading. All Centers agreed on the use of prednisone as first line treatment, which was started at the dose of 1 mg/kg in 26/33 Centers. Prednisone alone was used in 4 Centers, while in the others it was associated to extracorporeal photo-apheresis (ECP, N=25), calcineurine inhibitors (CNI, N=17) and mycophenolate mofetil (MMF, N=11). A great inter-center variety has been reported regarding the duration of treatment, as well as the indication to and the choice of steroid-sparing agents. All but 6 Centers referred to NIH criteria to define response. Objective measurements (i.e. pulmonary function and lab tests), patient reports and ability to treatment discontinuation were scored as of great importance for response judgment, whereas physician opinion was scored as medium. In case of complete response, 30/32 Centers tapered steroid slowly, but there was no uniformity on the definition of slow taper (9/32 agreed on 10% reduction/week). Treatment failure, steroid refractoriness, intolerance or dependency were the main reasons for second line therapy. Sixteen Centers stated to have a policy for the choice of second line treatment, and the choice was customized according to organ involvement and patients condition in 24/32 Centers. Seven Centers declared a policy for third line of treatment. Overall, CNI, ECP and MMF were the most used treatments for refractory cGVHD: CNI regardless the involved organ, ECP and sirolimus for skin, lung, and gastrointestinal (GI) involvement, imatinib for skin and lung, infliximab and MMF for liver and GI, and rituximab for skin. ECP was available in 25/32 Centers. Finally, all responding Centers reported a strong need and willing to participate in prospective multicenter trials for both first and second line treatment of cGVHD; only 2 of them had a protocol open for refractory GVHD.
Conclusion
Despite the presence of guidelines, this survey showed a great disparity in the management of cGVHD, especially for refractory disease. The survey further emphasized the great interest and need for prospective trials investigating this setting.
Session topic: E-poster
Keyword(s): Allogeneic stem cell transplant, Chronic graft-versus-host
Type: Eposter Presentation
Background
Diagnosing, scoring and treating chronic graft-versus-host disease (cGVHD) is challenging because of polymorphic manifestations and lack of biomarkers for the diagnosis and assessment of disease activity. Several guidelines have been published, however the clinical practice remains demanding.
Aims
With this study we investigated the 'real life' management of cGVHD in 32 Italian Center members of GITMO (Gruppo Italiano Trapianto Midollo Osseo).
Methods
A detailed survey with 41 multiple-choice questions about the use of guidelines, and the first and further lines of treatment and management of cGVHD has been proposed to all adult and pediatric GITMO Centers (N=60)
Results
Thirty-two Centers replied, and according to the survey's results, 29 Centers referred to published guidelines for cGVHD management (mainly those proposed by National Institute of Health, NIH), however only 13 find them fully adequate. As shown in Figure 1, the main reasons for complain were related to second line treatment. According to NIH definition, most of the Centers (N=25) started treatment when severe cGVHD occurred, whereas 4 Centers also considered bad prognostic features regardless the grading. All Centers agreed on the use of prednisone as first line treatment, which was started at the dose of 1 mg/kg in 26/33 Centers. Prednisone alone was used in 4 Centers, while in the others it was associated to extracorporeal photo-apheresis (ECP, N=25), calcineurine inhibitors (CNI, N=17) and mycophenolate mofetil (MMF, N=11). A great inter-center variety has been reported regarding the duration of treatment, as well as the indication to and the choice of steroid-sparing agents. All but 6 Centers referred to NIH criteria to define response. Objective measurements (i.e. pulmonary function and lab tests), patient reports and ability to treatment discontinuation were scored as of great importance for response judgment, whereas physician opinion was scored as medium. In case of complete response, 30/32 Centers tapered steroid slowly, but there was no uniformity on the definition of slow taper (9/32 agreed on 10% reduction/week). Treatment failure, steroid refractoriness, intolerance or dependency were the main reasons for second line therapy. Sixteen Centers stated to have a policy for the choice of second line treatment, and the choice was customized according to organ involvement and patients condition in 24/32 Centers. Seven Centers declared a policy for third line of treatment. Overall, CNI, ECP and MMF were the most used treatments for refractory cGVHD: CNI regardless the involved organ, ECP and sirolimus for skin, lung, and gastrointestinal (GI) involvement, imatinib for skin and lung, infliximab and MMF for liver and GI, and rituximab for skin. ECP was available in 25/32 Centers. Finally, all responding Centers reported a strong need and willing to participate in prospective multicenter trials for both first and second line treatment of cGVHD; only 2 of them had a protocol open for refractory GVHD.
Conclusion
Despite the presence of guidelines, this survey showed a great disparity in the management of cGVHD, especially for refractory disease. The survey further emphasized the great interest and need for prospective trials investigating this setting.

Session topic: E-poster
Keyword(s): Allogeneic stem cell transplant, Chronic graft-versus-host
Abstract: E1516
Type: Eposter Presentation
Background
Diagnosing, scoring and treating chronic graft-versus-host disease (cGVHD) is challenging because of polymorphic manifestations and lack of biomarkers for the diagnosis and assessment of disease activity. Several guidelines have been published, however the clinical practice remains demanding.
Aims
With this study we investigated the 'real life' management of cGVHD in 32 Italian Center members of GITMO (Gruppo Italiano Trapianto Midollo Osseo).
Methods
A detailed survey with 41 multiple-choice questions about the use of guidelines, and the first and further lines of treatment and management of cGVHD has been proposed to all adult and pediatric GITMO Centers (N=60)
Results
Thirty-two Centers replied, and according to the survey's results, 29 Centers referred to published guidelines for cGVHD management (mainly those proposed by National Institute of Health, NIH), however only 13 find them fully adequate. As shown in Figure 1, the main reasons for complain were related to second line treatment. According to NIH definition, most of the Centers (N=25) started treatment when severe cGVHD occurred, whereas 4 Centers also considered bad prognostic features regardless the grading. All Centers agreed on the use of prednisone as first line treatment, which was started at the dose of 1 mg/kg in 26/33 Centers. Prednisone alone was used in 4 Centers, while in the others it was associated to extracorporeal photo-apheresis (ECP, N=25), calcineurine inhibitors (CNI, N=17) and mycophenolate mofetil (MMF, N=11). A great inter-center variety has been reported regarding the duration of treatment, as well as the indication to and the choice of steroid-sparing agents. All but 6 Centers referred to NIH criteria to define response. Objective measurements (i.e. pulmonary function and lab tests), patient reports and ability to treatment discontinuation were scored as of great importance for response judgment, whereas physician opinion was scored as medium. In case of complete response, 30/32 Centers tapered steroid slowly, but there was no uniformity on the definition of slow taper (9/32 agreed on 10% reduction/week). Treatment failure, steroid refractoriness, intolerance or dependency were the main reasons for second line therapy. Sixteen Centers stated to have a policy for the choice of second line treatment, and the choice was customized according to organ involvement and patients condition in 24/32 Centers. Seven Centers declared a policy for third line of treatment. Overall, CNI, ECP and MMF were the most used treatments for refractory cGVHD: CNI regardless the involved organ, ECP and sirolimus for skin, lung, and gastrointestinal (GI) involvement, imatinib for skin and lung, infliximab and MMF for liver and GI, and rituximab for skin. ECP was available in 25/32 Centers. Finally, all responding Centers reported a strong need and willing to participate in prospective multicenter trials for both first and second line treatment of cGVHD; only 2 of them had a protocol open for refractory GVHD.
Conclusion
Despite the presence of guidelines, this survey showed a great disparity in the management of cGVHD, especially for refractory disease. The survey further emphasized the great interest and need for prospective trials investigating this setting.

Session topic: E-poster
Keyword(s): Allogeneic stem cell transplant, Chronic graft-versus-host
Type: Eposter Presentation
Background
Diagnosing, scoring and treating chronic graft-versus-host disease (cGVHD) is challenging because of polymorphic manifestations and lack of biomarkers for the diagnosis and assessment of disease activity. Several guidelines have been published, however the clinical practice remains demanding.
Aims
With this study we investigated the 'real life' management of cGVHD in 32 Italian Center members of GITMO (Gruppo Italiano Trapianto Midollo Osseo).
Methods
A detailed survey with 41 multiple-choice questions about the use of guidelines, and the first and further lines of treatment and management of cGVHD has been proposed to all adult and pediatric GITMO Centers (N=60)
Results
Thirty-two Centers replied, and according to the survey's results, 29 Centers referred to published guidelines for cGVHD management (mainly those proposed by National Institute of Health, NIH), however only 13 find them fully adequate. As shown in Figure 1, the main reasons for complain were related to second line treatment. According to NIH definition, most of the Centers (N=25) started treatment when severe cGVHD occurred, whereas 4 Centers also considered bad prognostic features regardless the grading. All Centers agreed on the use of prednisone as first line treatment, which was started at the dose of 1 mg/kg in 26/33 Centers. Prednisone alone was used in 4 Centers, while in the others it was associated to extracorporeal photo-apheresis (ECP, N=25), calcineurine inhibitors (CNI, N=17) and mycophenolate mofetil (MMF, N=11). A great inter-center variety has been reported regarding the duration of treatment, as well as the indication to and the choice of steroid-sparing agents. All but 6 Centers referred to NIH criteria to define response. Objective measurements (i.e. pulmonary function and lab tests), patient reports and ability to treatment discontinuation were scored as of great importance for response judgment, whereas physician opinion was scored as medium. In case of complete response, 30/32 Centers tapered steroid slowly, but there was no uniformity on the definition of slow taper (9/32 agreed on 10% reduction/week). Treatment failure, steroid refractoriness, intolerance or dependency were the main reasons for second line therapy. Sixteen Centers stated to have a policy for the choice of second line treatment, and the choice was customized according to organ involvement and patients condition in 24/32 Centers. Seven Centers declared a policy for third line of treatment. Overall, CNI, ECP and MMF were the most used treatments for refractory cGVHD: CNI regardless the involved organ, ECP and sirolimus for skin, lung, and gastrointestinal (GI) involvement, imatinib for skin and lung, infliximab and MMF for liver and GI, and rituximab for skin. ECP was available in 25/32 Centers. Finally, all responding Centers reported a strong need and willing to participate in prospective multicenter trials for both first and second line treatment of cGVHD; only 2 of them had a protocol open for refractory GVHD.
Conclusion
Despite the presence of guidelines, this survey showed a great disparity in the management of cGVHD, especially for refractory disease. The survey further emphasized the great interest and need for prospective trials investigating this setting.

Session topic: E-poster
Keyword(s): Allogeneic stem cell transplant, Chronic graft-versus-host
{{ help_message }}
{{filter}}


