A TWO-CENTER RETROSPECTIVE COMPARISON OF BEAM VS. BUCYVP16 CONDITIONING PRIOR TO AUTOLOGOUS HEMATOPOIETIC STEM CELL TRANSPLANTATION IN PATIENTS WITH HODGKIN’S LYMPHOMA
(Abstract release date: 05/19/16)
EHA Library. Singer S. 06/09/16; 133062; E1513

Dr. Sara Singer
Contributions
Contributions
Abstract
Abstract: E1513
Type: Eposter Presentation
Background
High dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) is an optional treatment for patients with relapsed Hodgkin’s Lymphoma (HL). A number of conditioning regimens are routinely used. However, the optimal conditioning regimen in terms of antitumor effect and toxicities remains unknown. We compare the outcomes of Carmustine, Etoposide, Cytarabine, Melphalan (BEAM) used at the Ohio State University to that of Busulfan, Cyclophosphamide, Etoposide (BUCYVP16) used at the Cleveland Clinic Foundation.
Aims
To compare the efficacy and toxicity of BEAM and BUCYVP16 conditioning regimens in patients with relapsed HL undergoing AHSCT.
Methods
We retrospectively analyzed patients treated with BEAM (n=128) or BUCYVP16 (n=105) followed by AHSCT between 2006 and 2014. Kaplan-Meier estimates were used to analyze progression free survival (PFS) and overall survival (OS). Cumulative incidence of relapse (CIR) was measured from transplant date until relapse, treating deaths as competing risks using Pepe and Mori test.
Results
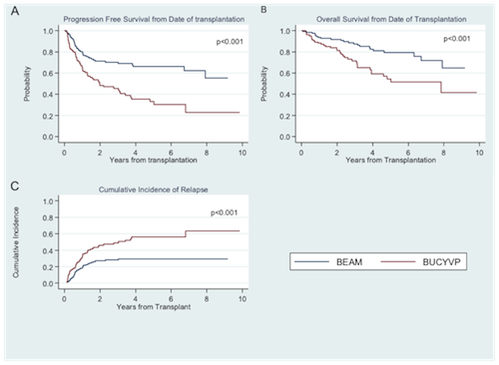
Patient characteristics were similar for age (median 34 (19-73) vs. 38 (19-69), p=0.51), disease stage (p=0.17), prior radiation exposure (p=0.21), comorbidity index (p=0.58), and response status at transplant (89.1% CR/PR vs. 90.3%) for BEAM vs. BUCYVP16 respectively. Differences were number of prior treatments (median 3 (1-10) vs. 2 (1-5), p<0.01), and median CD34 dose infused (4.45 x10^6 vs. 7.25, p<0.01) for BEAM vs. BUCYVP16. Median follow-up from diagnosis and transplant was 7.0 years and 4.2 years respectively for BEAM and 6.5 years and 3.8 years for BUCYVP16. Median day to neutrophil engraftment was 10 (8-13) vs. 10 (9-12) (p<0.01) and median time to platelet engraftment was 18 (13-70) vs. 16 (7-49) (p<0.01) for BEAM vs. BUCYVP16. Patients receiving BEAM had a lower CIR at 1, 3, and 5 years compared to BUCYVP16 (p < 0.001). PFS at 1, 3 and 5 years from HSCT was 80%, 70% and 66% for BEAM vs. 65%, 45% and 33% for BUCYVP16 respectively (P<0.001). OS from HSCT at 1, 3, and 5 years was also longer in the BEAM cohort: 94%, 88%, and 79% compared to 88%, 72%, and 54% in the BUCVY16 cohort (p<0.001, Figure 1). Grade 3/4 mucositis was higher for patients receiving BUCYVP16 (p=0.01). There were no differences in hemorrhagic cystitis, veno-occlusive disease or second primary malignancy.
Conclusion
This large retrospective study supports the use of BEAM as a conditioning regimen for AHSCT for relapsed HL. Both PFS and OS were longer for the BEAM cohort with a lower CIR.
Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Busulfan, Hodgkin's lymphoma, Melphalan
Type: Eposter Presentation
Background
High dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) is an optional treatment for patients with relapsed Hodgkin’s Lymphoma (HL). A number of conditioning regimens are routinely used. However, the optimal conditioning regimen in terms of antitumor effect and toxicities remains unknown. We compare the outcomes of Carmustine, Etoposide, Cytarabine, Melphalan (BEAM) used at the Ohio State University to that of Busulfan, Cyclophosphamide, Etoposide (BUCYVP16) used at the Cleveland Clinic Foundation.
Aims
To compare the efficacy and toxicity of BEAM and BUCYVP16 conditioning regimens in patients with relapsed HL undergoing AHSCT.
Methods
We retrospectively analyzed patients treated with BEAM (n=128) or BUCYVP16 (n=105) followed by AHSCT between 2006 and 2014. Kaplan-Meier estimates were used to analyze progression free survival (PFS) and overall survival (OS). Cumulative incidence of relapse (CIR) was measured from transplant date until relapse, treating deaths as competing risks using Pepe and Mori test.
Results
Patient characteristics were similar for age (median 34 (19-73) vs. 38 (19-69), p=0.51), disease stage (p=0.17), prior radiation exposure (p=0.21), comorbidity index (p=0.58), and response status at transplant (89.1% CR/PR vs. 90.3%) for BEAM vs. BUCYVP16 respectively. Differences were number of prior treatments (median 3 (1-10) vs. 2 (1-5), p<0.01), and median CD34 dose infused (4.45 x10^6 vs. 7.25, p<0.01) for BEAM vs. BUCYVP16. Median follow-up from diagnosis and transplant was 7.0 years and 4.2 years respectively for BEAM and 6.5 years and 3.8 years for BUCYVP16. Median day to neutrophil engraftment was 10 (8-13) vs. 10 (9-12) (p<0.01) and median time to platelet engraftment was 18 (13-70) vs. 16 (7-49) (p<0.01) for BEAM vs. BUCYVP16. Patients receiving BEAM had a lower CIR at 1, 3, and 5 years compared to BUCYVP16 (p < 0.001). PFS at 1, 3 and 5 years from HSCT was 80%, 70% and 66% for BEAM vs. 65%, 45% and 33% for BUCYVP16 respectively (P<0.001). OS from HSCT at 1, 3, and 5 years was also longer in the BEAM cohort: 94%, 88%, and 79% compared to 88%, 72%, and 54% in the BUCVY16 cohort (p<0.001, Figure 1). Grade 3/4 mucositis was higher for patients receiving BUCYVP16 (p=0.01). There were no differences in hemorrhagic cystitis, veno-occlusive disease or second primary malignancy.
Conclusion
This large retrospective study supports the use of BEAM as a conditioning regimen for AHSCT for relapsed HL. Both PFS and OS were longer for the BEAM cohort with a lower CIR.

Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Busulfan, Hodgkin's lymphoma, Melphalan
Abstract: E1513
Type: Eposter Presentation
Background
High dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) is an optional treatment for patients with relapsed Hodgkin’s Lymphoma (HL). A number of conditioning regimens are routinely used. However, the optimal conditioning regimen in terms of antitumor effect and toxicities remains unknown. We compare the outcomes of Carmustine, Etoposide, Cytarabine, Melphalan (BEAM) used at the Ohio State University to that of Busulfan, Cyclophosphamide, Etoposide (BUCYVP16) used at the Cleveland Clinic Foundation.
Aims
To compare the efficacy and toxicity of BEAM and BUCYVP16 conditioning regimens in patients with relapsed HL undergoing AHSCT.
Methods
We retrospectively analyzed patients treated with BEAM (n=128) or BUCYVP16 (n=105) followed by AHSCT between 2006 and 2014. Kaplan-Meier estimates were used to analyze progression free survival (PFS) and overall survival (OS). Cumulative incidence of relapse (CIR) was measured from transplant date until relapse, treating deaths as competing risks using Pepe and Mori test.
Results
Patient characteristics were similar for age (median 34 (19-73) vs. 38 (19-69), p=0.51), disease stage (p=0.17), prior radiation exposure (p=0.21), comorbidity index (p=0.58), and response status at transplant (89.1% CR/PR vs. 90.3%) for BEAM vs. BUCYVP16 respectively. Differences were number of prior treatments (median 3 (1-10) vs. 2 (1-5), p<0.01), and median CD34 dose infused (4.45 x10^6 vs. 7.25, p<0.01) for BEAM vs. BUCYVP16. Median follow-up from diagnosis and transplant was 7.0 years and 4.2 years respectively for BEAM and 6.5 years and 3.8 years for BUCYVP16. Median day to neutrophil engraftment was 10 (8-13) vs. 10 (9-12) (p<0.01) and median time to platelet engraftment was 18 (13-70) vs. 16 (7-49) (p<0.01) for BEAM vs. BUCYVP16. Patients receiving BEAM had a lower CIR at 1, 3, and 5 years compared to BUCYVP16 (p < 0.001). PFS at 1, 3 and 5 years from HSCT was 80%, 70% and 66% for BEAM vs. 65%, 45% and 33% for BUCYVP16 respectively (P<0.001). OS from HSCT at 1, 3, and 5 years was also longer in the BEAM cohort: 94%, 88%, and 79% compared to 88%, 72%, and 54% in the BUCVY16 cohort (p<0.001, Figure 1). Grade 3/4 mucositis was higher for patients receiving BUCYVP16 (p=0.01). There were no differences in hemorrhagic cystitis, veno-occlusive disease or second primary malignancy.
Conclusion
This large retrospective study supports the use of BEAM as a conditioning regimen for AHSCT for relapsed HL. Both PFS and OS were longer for the BEAM cohort with a lower CIR.

Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Busulfan, Hodgkin's lymphoma, Melphalan
Type: Eposter Presentation
Background
High dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) is an optional treatment for patients with relapsed Hodgkin’s Lymphoma (HL). A number of conditioning regimens are routinely used. However, the optimal conditioning regimen in terms of antitumor effect and toxicities remains unknown. We compare the outcomes of Carmustine, Etoposide, Cytarabine, Melphalan (BEAM) used at the Ohio State University to that of Busulfan, Cyclophosphamide, Etoposide (BUCYVP16) used at the Cleveland Clinic Foundation.
Aims
To compare the efficacy and toxicity of BEAM and BUCYVP16 conditioning regimens in patients with relapsed HL undergoing AHSCT.
Methods
We retrospectively analyzed patients treated with BEAM (n=128) or BUCYVP16 (n=105) followed by AHSCT between 2006 and 2014. Kaplan-Meier estimates were used to analyze progression free survival (PFS) and overall survival (OS). Cumulative incidence of relapse (CIR) was measured from transplant date until relapse, treating deaths as competing risks using Pepe and Mori test.
Results
Patient characteristics were similar for age (median 34 (19-73) vs. 38 (19-69), p=0.51), disease stage (p=0.17), prior radiation exposure (p=0.21), comorbidity index (p=0.58), and response status at transplant (89.1% CR/PR vs. 90.3%) for BEAM vs. BUCYVP16 respectively. Differences were number of prior treatments (median 3 (1-10) vs. 2 (1-5), p<0.01), and median CD34 dose infused (4.45 x10^6 vs. 7.25, p<0.01) for BEAM vs. BUCYVP16. Median follow-up from diagnosis and transplant was 7.0 years and 4.2 years respectively for BEAM and 6.5 years and 3.8 years for BUCYVP16. Median day to neutrophil engraftment was 10 (8-13) vs. 10 (9-12) (p<0.01) and median time to platelet engraftment was 18 (13-70) vs. 16 (7-49) (p<0.01) for BEAM vs. BUCYVP16. Patients receiving BEAM had a lower CIR at 1, 3, and 5 years compared to BUCYVP16 (p < 0.001). PFS at 1, 3 and 5 years from HSCT was 80%, 70% and 66% for BEAM vs. 65%, 45% and 33% for BUCYVP16 respectively (P<0.001). OS from HSCT at 1, 3, and 5 years was also longer in the BEAM cohort: 94%, 88%, and 79% compared to 88%, 72%, and 54% in the BUCVY16 cohort (p<0.001, Figure 1). Grade 3/4 mucositis was higher for patients receiving BUCYVP16 (p=0.01). There were no differences in hemorrhagic cystitis, veno-occlusive disease or second primary malignancy.
Conclusion
This large retrospective study supports the use of BEAM as a conditioning regimen for AHSCT for relapsed HL. Both PFS and OS were longer for the BEAM cohort with a lower CIR.

Session topic: E-poster
Keyword(s): Autologous hematopoietic stem cell transplantation, Busulfan, Hodgkin's lymphoma, Melphalan
{{ help_message }}
{{filter}}


