IMPACT OF T CELL DOSE ON OUTCOMES OF NON-MYELOABLATIVE ALLOGENEIC TRANSPLANTS IN MULTIPLE MYELOMA
(Abstract release date: 05/19/16)
EHA Library. Puliyayil A. 06/09/16; 133048; E1499

Dr. Anish Puliyayil
Contributions
Contributions
Abstract
Abstract: E1499
Type: Eposter Presentation
Background
Donor T cells are responsible for graft versus host disease (GVHD) following allogeneic stem cell transplantation (alloSCT). Studies have shown a reduced incidence of GVHD but increased relapse risk with T cell depleted transplants. T cell replete stem cells are generally used for non-myeloablative (NMA) alloSCT to prevent graft failure and to avail the benefit of a potential graft versus tumor effect which is considered the major tool for disease control in this type of transplant. T cell doses in individual T cell replete transplants vary and to the best of our knowledge no previous studies have looked at the impact of T cell dose on the outcome of NMA alloSCT.We routinely perform tandem autologous (ASCT)-NMA alloSCT for high risk as well as relapsed multiple myeloma (MM) patients at our centre. High risk MM patients are defined by the presence of at least 2 out of 5 risk features including International Staging System score 3, adverse cytogenetics [t(4;14),17p- on FISH and/or complex karyotype], elevated lactate dehydrogenase, plasma cell leukemia (all at diagnosis) and induction failure (
Aims
To find out whether the outcomes after NMA SCT for MM were influenced by the T cell dose received.
Methods
After obtaining informed consent, we undertook a retrospective analysis of patients who underwent tandem ASCT-NMA SCT from May 2008 to June 2015 for MM. Primary end points were progression free survival (PFS) and overall survival (OS). Secondary end points were cumulative incidences of acute GVHD, chronic GVHD and relapse, treatment related mortality and achievement of donor chimerism.
Results
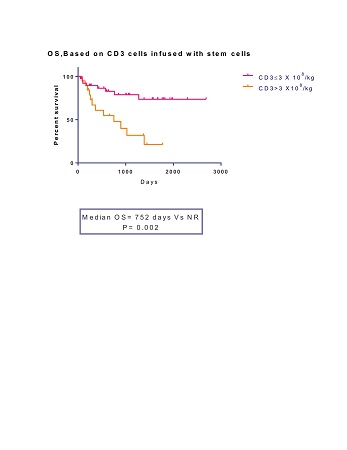
Out of 59, 19 patients had received T cell dose of ≥3 X 108/kg. This high dose cohort (Hi-T) was compared to 40 patients who got <3 X108/kg T cells (Lo-T). Median age was 59yrs (range 22-66yrs) for Hi-T and 54yrs (range 38-67yrs) for Lo-T (p=0.43). 52.5% of patients received upfront transplants in Hi-T against 42% in Lo-T (p=0.23). 68% of patients had unrelated donors in Hi-T versus 57.5% in Lo-T (p=0.22). After a median follow up of 45.7 months, PFS and OS were significantly inferior in Hi-T group (median PFS 366 days vs 1067 days, p=0.05, median OS 752 days vs not reached, p=0.002). Cumulative incidences of grade 2-4 acute GVHD and extensive chronic GVHD were higher in Hi-T (47% vs 17.5%; p= 0.03 and 80% vs 50%; p= 0.04 respectively). There were 5 treatment related deaths (26.3%) in Hi-T (all due to GVHD) against 2 (5%) in Lo-T (both due to infections); (p=0.009). Cumulative incidence of relapse was not different between the arms (48% vs 49% respectively at 4yrs.). Achievement of donor CD3 chimerism at different time points (days 30, 60, 90 and 180) was not different between the arms. All patients who were alive at 1yr achieved full donor chimerism. On multivariate analysis, Hi-T was independently associated with inferior OS.
Conclusion
To conclude, in NMA alloSCT for MM, the T cell dose infused has a significant influence on outcomes. Limiting the T cell dose to <3 X 108/kg can improve the OS and reduce TRM and GVHD. Larger randomised trials are required to confirm this observation.
Session topic: E-poster
Keyword(s): Multiple myeloma, Non myeloablative stem cell transplant, T cell
Type: Eposter Presentation
Background
Donor T cells are responsible for graft versus host disease (GVHD) following allogeneic stem cell transplantation (alloSCT). Studies have shown a reduced incidence of GVHD but increased relapse risk with T cell depleted transplants. T cell replete stem cells are generally used for non-myeloablative (NMA) alloSCT to prevent graft failure and to avail the benefit of a potential graft versus tumor effect which is considered the major tool for disease control in this type of transplant. T cell doses in individual T cell replete transplants vary and to the best of our knowledge no previous studies have looked at the impact of T cell dose on the outcome of NMA alloSCT.We routinely perform tandem autologous (ASCT)-NMA alloSCT for high risk as well as relapsed multiple myeloma (MM) patients at our centre. High risk MM patients are defined by the presence of at least 2 out of 5 risk features including International Staging System score 3, adverse cytogenetics [t(4;14),17p- on FISH and/or complex karyotype], elevated lactate dehydrogenase, plasma cell leukemia (all at diagnosis) and induction failure (
Aims
To find out whether the outcomes after NMA SCT for MM were influenced by the T cell dose received.
Methods
After obtaining informed consent, we undertook a retrospective analysis of patients who underwent tandem ASCT-NMA SCT from May 2008 to June 2015 for MM. Primary end points were progression free survival (PFS) and overall survival (OS). Secondary end points were cumulative incidences of acute GVHD, chronic GVHD and relapse, treatment related mortality and achievement of donor chimerism.
Results
Out of 59, 19 patients had received T cell dose of ≥3 X 108/kg. This high dose cohort (Hi-T) was compared to 40 patients who got <3 X108/kg T cells (Lo-T). Median age was 59yrs (range 22-66yrs) for Hi-T and 54yrs (range 38-67yrs) for Lo-T (p=0.43). 52.5% of patients received upfront transplants in Hi-T against 42% in Lo-T (p=0.23). 68% of patients had unrelated donors in Hi-T versus 57.5% in Lo-T (p=0.22). After a median follow up of 45.7 months, PFS and OS were significantly inferior in Hi-T group (median PFS 366 days vs 1067 days, p=0.05, median OS 752 days vs not reached, p=0.002). Cumulative incidences of grade 2-4 acute GVHD and extensive chronic GVHD were higher in Hi-T (47% vs 17.5%; p= 0.03 and 80% vs 50%; p= 0.04 respectively). There were 5 treatment related deaths (26.3%) in Hi-T (all due to GVHD) against 2 (5%) in Lo-T (both due to infections); (p=0.009). Cumulative incidence of relapse was not different between the arms (48% vs 49% respectively at 4yrs.). Achievement of donor CD3 chimerism at different time points (days 30, 60, 90 and 180) was not different between the arms. All patients who were alive at 1yr achieved full donor chimerism. On multivariate analysis, Hi-T was independently associated with inferior OS.
Conclusion
To conclude, in NMA alloSCT for MM, the T cell dose infused has a significant influence on outcomes. Limiting the T cell dose to <3 X 108/kg can improve the OS and reduce TRM and GVHD. Larger randomised trials are required to confirm this observation.

Session topic: E-poster
Keyword(s): Multiple myeloma, Non myeloablative stem cell transplant, T cell
Abstract: E1499
Type: Eposter Presentation
Background
Donor T cells are responsible for graft versus host disease (GVHD) following allogeneic stem cell transplantation (alloSCT). Studies have shown a reduced incidence of GVHD but increased relapse risk with T cell depleted transplants. T cell replete stem cells are generally used for non-myeloablative (NMA) alloSCT to prevent graft failure and to avail the benefit of a potential graft versus tumor effect which is considered the major tool for disease control in this type of transplant. T cell doses in individual T cell replete transplants vary and to the best of our knowledge no previous studies have looked at the impact of T cell dose on the outcome of NMA alloSCT.We routinely perform tandem autologous (ASCT)-NMA alloSCT for high risk as well as relapsed multiple myeloma (MM) patients at our centre. High risk MM patients are defined by the presence of at least 2 out of 5 risk features including International Staging System score 3, adverse cytogenetics [t(4;14),17p- on FISH and/or complex karyotype], elevated lactate dehydrogenase, plasma cell leukemia (all at diagnosis) and induction failure (
Aims
To find out whether the outcomes after NMA SCT for MM were influenced by the T cell dose received.
Methods
After obtaining informed consent, we undertook a retrospective analysis of patients who underwent tandem ASCT-NMA SCT from May 2008 to June 2015 for MM. Primary end points were progression free survival (PFS) and overall survival (OS). Secondary end points were cumulative incidences of acute GVHD, chronic GVHD and relapse, treatment related mortality and achievement of donor chimerism.
Results
Out of 59, 19 patients had received T cell dose of ≥3 X 108/kg. This high dose cohort (Hi-T) was compared to 40 patients who got <3 X108/kg T cells (Lo-T). Median age was 59yrs (range 22-66yrs) for Hi-T and 54yrs (range 38-67yrs) for Lo-T (p=0.43). 52.5% of patients received upfront transplants in Hi-T against 42% in Lo-T (p=0.23). 68% of patients had unrelated donors in Hi-T versus 57.5% in Lo-T (p=0.22). After a median follow up of 45.7 months, PFS and OS were significantly inferior in Hi-T group (median PFS 366 days vs 1067 days, p=0.05, median OS 752 days vs not reached, p=0.002). Cumulative incidences of grade 2-4 acute GVHD and extensive chronic GVHD were higher in Hi-T (47% vs 17.5%; p= 0.03 and 80% vs 50%; p= 0.04 respectively). There were 5 treatment related deaths (26.3%) in Hi-T (all due to GVHD) against 2 (5%) in Lo-T (both due to infections); (p=0.009). Cumulative incidence of relapse was not different between the arms (48% vs 49% respectively at 4yrs.). Achievement of donor CD3 chimerism at different time points (days 30, 60, 90 and 180) was not different between the arms. All patients who were alive at 1yr achieved full donor chimerism. On multivariate analysis, Hi-T was independently associated with inferior OS.
Conclusion
To conclude, in NMA alloSCT for MM, the T cell dose infused has a significant influence on outcomes. Limiting the T cell dose to <3 X 108/kg can improve the OS and reduce TRM and GVHD. Larger randomised trials are required to confirm this observation.

Session topic: E-poster
Keyword(s): Multiple myeloma, Non myeloablative stem cell transplant, T cell
Type: Eposter Presentation
Background
Donor T cells are responsible for graft versus host disease (GVHD) following allogeneic stem cell transplantation (alloSCT). Studies have shown a reduced incidence of GVHD but increased relapse risk with T cell depleted transplants. T cell replete stem cells are generally used for non-myeloablative (NMA) alloSCT to prevent graft failure and to avail the benefit of a potential graft versus tumor effect which is considered the major tool for disease control in this type of transplant. T cell doses in individual T cell replete transplants vary and to the best of our knowledge no previous studies have looked at the impact of T cell dose on the outcome of NMA alloSCT.We routinely perform tandem autologous (ASCT)-NMA alloSCT for high risk as well as relapsed multiple myeloma (MM) patients at our centre. High risk MM patients are defined by the presence of at least 2 out of 5 risk features including International Staging System score 3, adverse cytogenetics [t(4;14),17p- on FISH and/or complex karyotype], elevated lactate dehydrogenase, plasma cell leukemia (all at diagnosis) and induction failure (
Aims
To find out whether the outcomes after NMA SCT for MM were influenced by the T cell dose received.
Methods
After obtaining informed consent, we undertook a retrospective analysis of patients who underwent tandem ASCT-NMA SCT from May 2008 to June 2015 for MM. Primary end points were progression free survival (PFS) and overall survival (OS). Secondary end points were cumulative incidences of acute GVHD, chronic GVHD and relapse, treatment related mortality and achievement of donor chimerism.
Results
Out of 59, 19 patients had received T cell dose of ≥3 X 108/kg. This high dose cohort (Hi-T) was compared to 40 patients who got <3 X108/kg T cells (Lo-T). Median age was 59yrs (range 22-66yrs) for Hi-T and 54yrs (range 38-67yrs) for Lo-T (p=0.43). 52.5% of patients received upfront transplants in Hi-T against 42% in Lo-T (p=0.23). 68% of patients had unrelated donors in Hi-T versus 57.5% in Lo-T (p=0.22). After a median follow up of 45.7 months, PFS and OS were significantly inferior in Hi-T group (median PFS 366 days vs 1067 days, p=0.05, median OS 752 days vs not reached, p=0.002). Cumulative incidences of grade 2-4 acute GVHD and extensive chronic GVHD were higher in Hi-T (47% vs 17.5%; p= 0.03 and 80% vs 50%; p= 0.04 respectively). There were 5 treatment related deaths (26.3%) in Hi-T (all due to GVHD) against 2 (5%) in Lo-T (both due to infections); (p=0.009). Cumulative incidence of relapse was not different between the arms (48% vs 49% respectively at 4yrs.). Achievement of donor CD3 chimerism at different time points (days 30, 60, 90 and 180) was not different between the arms. All patients who were alive at 1yr achieved full donor chimerism. On multivariate analysis, Hi-T was independently associated with inferior OS.
Conclusion
To conclude, in NMA alloSCT for MM, the T cell dose infused has a significant influence on outcomes. Limiting the T cell dose to <3 X 108/kg can improve the OS and reduce TRM and GVHD. Larger randomised trials are required to confirm this observation.

Session topic: E-poster
Keyword(s): Multiple myeloma, Non myeloablative stem cell transplant, T cell
{{ help_message }}
{{filter}}


