PROHEPCIDIN CONCENTRATIONS IN GAUCHER DISEASE AND FABRY DISEASE: INVESTIGATING THE ROLE OF HEPCIDIN IN ABNORMAL IRON HOMEOSTASIS AND ANAEMIA
(Abstract release date: 05/19/16)
EHA Library. Creamer A. 06/09/16; 133045; E1496

Dr. Andrew Creamer
Contributions
Contributions
Abstract
Abstract: E1496
Type: Eposter Presentation
Background
Hepcidin is a 25-amino acid peptide first identified in 2001. It is recognised to have a key role in the regulation of iron stores, acting on duodenal enterocytes, macrophages and hepatocytes. Hepcidin levels have been found to respond to inflammatory cytokines (IL-6), iron status, erythropoetic activity and oxygen tension. Its precursor, prohepcidin, is synthesised by the liver and can be measured by a commercially available ELISA. Gaucher disease (GD) and Anderson-Fabry disease (AFD) are lysosomal storage disorders, a heterogenous group of diseases resulting from an inherited defect in a specific lysosomal enzyme or associated proteins. Haematological abnormalities including anaemia and disordered iron homestasis are recognised in GD. AFD is more commonly associated with organ dysfunction, but anaemia has also been described.
Aims
The Lysosomal Storage Disorders Unit at the Royal Free Hospital, London, has a unique cohort of patients with GD and AFD. By measuring serum prohepcidin levels, along with haematological indices and levels of circulating inflammatory cytokines, we sought to investigate the role of hepcidin/prohepcidin in GD and AFD. We looked specifically at whether it may have a role in the pathophysiology of the disordered iron profile and anaemia associated with GD.
Methods
Consecutive samples from patients with GD (n=33) or AFD (n=41) and normal control subjects (n=10) were obtained. All patients gave informed consent. Commercial ELISA kits were used to measure soluble transferrin receptor (Biovendor Medicine Inc) and IL-6 (BD Biosciences). A commercial ELISA for prohepcidin (DRG International Inc) was used.
Results
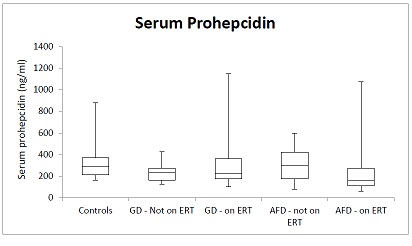
A total of 33 patients with GD were included, with 27 receiving enzyme replacement therapy (ERT). A total of N= 41 patients with AFD were included, of which 26 were receiving ERT.All groups had mean haemoglobin (Hb) within normal reference ranges. Mean soluble transferrin receptor (sTfR) levels were below lab reference ranges (1.8-4.6mg/L) for both ERT and non-ERT receiving patients in GD and AFD groups (GD on ERT mean 1.51mg/L, no ERT 1.47mg.L, AFD on ERT mean 1.04 mg/L, no ERT 0.93mg/L). GD patients had mean ferritin levels elevated above the upper limit of normal reference range (Non-ERT group mean = 453 µg/L, ERT group mean 398 µg/L). For AFD patients, mean ferritin was within in the normal range for both ERT (88 µg/L) and non-ERT (54 µg/L) receiving groups. In all groups, there was a wide range of serum prohepcidin levels (figure 1). Mean prohepcidin was lower in both AFD and GD groups vs controls, but this did not reach statistical significance in any group. There was no correlation between prohepcidin and Hb, serum IL-6 or serum sTfR in any group.
Conclusion
Our study confirms previous results that GD is associated with a hyperferritinaemic state which is not found in AFD. As sTfR levels in GD and AFD patients were low, this appears to be secondary to inflammation rather than iron status. As in previous studies, we found IL-6 levels to be higher in GD patients then controls. Anaemia was not prevalent in any patient groups, although this may be masked by treatment and appropriate transfusion in a carefully monitored patient group. There was no significant difference between prohepcidin levels in AFD or GD patients and controls, and no correlation between Hb, IL-6, or iron status with prohepcidin in any group. The extent to which prohepcidin reflects hepcidin itself is controversial, but there is a growing body of evidence that prohepcidin levels are affected by inflammatory conditions, iron deficiency anaemia and changes in EPO levels, amongst other things. Further studies, with a higher number of patients, and using an ELISA targeted against hepcidin itself are required to further explore what role it may play in AFD/GD patients.
Session topic: E-poster
Keyword(s): Gaucher disease, Iron, Lysosomal storage disease
Type: Eposter Presentation
Background
Hepcidin is a 25-amino acid peptide first identified in 2001. It is recognised to have a key role in the regulation of iron stores, acting on duodenal enterocytes, macrophages and hepatocytes. Hepcidin levels have been found to respond to inflammatory cytokines (IL-6), iron status, erythropoetic activity and oxygen tension. Its precursor, prohepcidin, is synthesised by the liver and can be measured by a commercially available ELISA. Gaucher disease (GD) and Anderson-Fabry disease (AFD) are lysosomal storage disorders, a heterogenous group of diseases resulting from an inherited defect in a specific lysosomal enzyme or associated proteins. Haematological abnormalities including anaemia and disordered iron homestasis are recognised in GD. AFD is more commonly associated with organ dysfunction, but anaemia has also been described.
Aims
The Lysosomal Storage Disorders Unit at the Royal Free Hospital, London, has a unique cohort of patients with GD and AFD. By measuring serum prohepcidin levels, along with haematological indices and levels of circulating inflammatory cytokines, we sought to investigate the role of hepcidin/prohepcidin in GD and AFD. We looked specifically at whether it may have a role in the pathophysiology of the disordered iron profile and anaemia associated with GD.
Methods
Consecutive samples from patients with GD (n=33) or AFD (n=41) and normal control subjects (n=10) were obtained. All patients gave informed consent. Commercial ELISA kits were used to measure soluble transferrin receptor (Biovendor Medicine Inc) and IL-6 (BD Biosciences). A commercial ELISA for prohepcidin (DRG International Inc) was used.
Results
A total of 33 patients with GD were included, with 27 receiving enzyme replacement therapy (ERT). A total of N= 41 patients with AFD were included, of which 26 were receiving ERT.All groups had mean haemoglobin (Hb) within normal reference ranges. Mean soluble transferrin receptor (sTfR) levels were below lab reference ranges (1.8-4.6mg/L) for both ERT and non-ERT receiving patients in GD and AFD groups (GD on ERT mean 1.51mg/L, no ERT 1.47mg.L, AFD on ERT mean 1.04 mg/L, no ERT 0.93mg/L). GD patients had mean ferritin levels elevated above the upper limit of normal reference range (Non-ERT group mean = 453 µg/L, ERT group mean 398 µg/L). For AFD patients, mean ferritin was within in the normal range for both ERT (88 µg/L) and non-ERT (54 µg/L) receiving groups. In all groups, there was a wide range of serum prohepcidin levels (figure 1). Mean prohepcidin was lower in both AFD and GD groups vs controls, but this did not reach statistical significance in any group. There was no correlation between prohepcidin and Hb, serum IL-6 or serum sTfR in any group.
Conclusion
Our study confirms previous results that GD is associated with a hyperferritinaemic state which is not found in AFD. As sTfR levels in GD and AFD patients were low, this appears to be secondary to inflammation rather than iron status. As in previous studies, we found IL-6 levels to be higher in GD patients then controls. Anaemia was not prevalent in any patient groups, although this may be masked by treatment and appropriate transfusion in a carefully monitored patient group. There was no significant difference between prohepcidin levels in AFD or GD patients and controls, and no correlation between Hb, IL-6, or iron status with prohepcidin in any group. The extent to which prohepcidin reflects hepcidin itself is controversial, but there is a growing body of evidence that prohepcidin levels are affected by inflammatory conditions, iron deficiency anaemia and changes in EPO levels, amongst other things. Further studies, with a higher number of patients, and using an ELISA targeted against hepcidin itself are required to further explore what role it may play in AFD/GD patients.

Session topic: E-poster
Keyword(s): Gaucher disease, Iron, Lysosomal storage disease
Abstract: E1496
Type: Eposter Presentation
Background
Hepcidin is a 25-amino acid peptide first identified in 2001. It is recognised to have a key role in the regulation of iron stores, acting on duodenal enterocytes, macrophages and hepatocytes. Hepcidin levels have been found to respond to inflammatory cytokines (IL-6), iron status, erythropoetic activity and oxygen tension. Its precursor, prohepcidin, is synthesised by the liver and can be measured by a commercially available ELISA. Gaucher disease (GD) and Anderson-Fabry disease (AFD) are lysosomal storage disorders, a heterogenous group of diseases resulting from an inherited defect in a specific lysosomal enzyme or associated proteins. Haematological abnormalities including anaemia and disordered iron homestasis are recognised in GD. AFD is more commonly associated with organ dysfunction, but anaemia has also been described.
Aims
The Lysosomal Storage Disorders Unit at the Royal Free Hospital, London, has a unique cohort of patients with GD and AFD. By measuring serum prohepcidin levels, along with haematological indices and levels of circulating inflammatory cytokines, we sought to investigate the role of hepcidin/prohepcidin in GD and AFD. We looked specifically at whether it may have a role in the pathophysiology of the disordered iron profile and anaemia associated with GD.
Methods
Consecutive samples from patients with GD (n=33) or AFD (n=41) and normal control subjects (n=10) were obtained. All patients gave informed consent. Commercial ELISA kits were used to measure soluble transferrin receptor (Biovendor Medicine Inc) and IL-6 (BD Biosciences). A commercial ELISA for prohepcidin (DRG International Inc) was used.
Results
A total of 33 patients with GD were included, with 27 receiving enzyme replacement therapy (ERT). A total of N= 41 patients with AFD were included, of which 26 were receiving ERT.All groups had mean haemoglobin (Hb) within normal reference ranges. Mean soluble transferrin receptor (sTfR) levels were below lab reference ranges (1.8-4.6mg/L) for both ERT and non-ERT receiving patients in GD and AFD groups (GD on ERT mean 1.51mg/L, no ERT 1.47mg.L, AFD on ERT mean 1.04 mg/L, no ERT 0.93mg/L). GD patients had mean ferritin levels elevated above the upper limit of normal reference range (Non-ERT group mean = 453 µg/L, ERT group mean 398 µg/L). For AFD patients, mean ferritin was within in the normal range for both ERT (88 µg/L) and non-ERT (54 µg/L) receiving groups. In all groups, there was a wide range of serum prohepcidin levels (figure 1). Mean prohepcidin was lower in both AFD and GD groups vs controls, but this did not reach statistical significance in any group. There was no correlation between prohepcidin and Hb, serum IL-6 or serum sTfR in any group.
Conclusion
Our study confirms previous results that GD is associated with a hyperferritinaemic state which is not found in AFD. As sTfR levels in GD and AFD patients were low, this appears to be secondary to inflammation rather than iron status. As in previous studies, we found IL-6 levels to be higher in GD patients then controls. Anaemia was not prevalent in any patient groups, although this may be masked by treatment and appropriate transfusion in a carefully monitored patient group. There was no significant difference between prohepcidin levels in AFD or GD patients and controls, and no correlation between Hb, IL-6, or iron status with prohepcidin in any group. The extent to which prohepcidin reflects hepcidin itself is controversial, but there is a growing body of evidence that prohepcidin levels are affected by inflammatory conditions, iron deficiency anaemia and changes in EPO levels, amongst other things. Further studies, with a higher number of patients, and using an ELISA targeted against hepcidin itself are required to further explore what role it may play in AFD/GD patients.

Session topic: E-poster
Keyword(s): Gaucher disease, Iron, Lysosomal storage disease
Type: Eposter Presentation
Background
Hepcidin is a 25-amino acid peptide first identified in 2001. It is recognised to have a key role in the regulation of iron stores, acting on duodenal enterocytes, macrophages and hepatocytes. Hepcidin levels have been found to respond to inflammatory cytokines (IL-6), iron status, erythropoetic activity and oxygen tension. Its precursor, prohepcidin, is synthesised by the liver and can be measured by a commercially available ELISA. Gaucher disease (GD) and Anderson-Fabry disease (AFD) are lysosomal storage disorders, a heterogenous group of diseases resulting from an inherited defect in a specific lysosomal enzyme or associated proteins. Haematological abnormalities including anaemia and disordered iron homestasis are recognised in GD. AFD is more commonly associated with organ dysfunction, but anaemia has also been described.
Aims
The Lysosomal Storage Disorders Unit at the Royal Free Hospital, London, has a unique cohort of patients with GD and AFD. By measuring serum prohepcidin levels, along with haematological indices and levels of circulating inflammatory cytokines, we sought to investigate the role of hepcidin/prohepcidin in GD and AFD. We looked specifically at whether it may have a role in the pathophysiology of the disordered iron profile and anaemia associated with GD.
Methods
Consecutive samples from patients with GD (n=33) or AFD (n=41) and normal control subjects (n=10) were obtained. All patients gave informed consent. Commercial ELISA kits were used to measure soluble transferrin receptor (Biovendor Medicine Inc) and IL-6 (BD Biosciences). A commercial ELISA for prohepcidin (DRG International Inc) was used.
Results
A total of 33 patients with GD were included, with 27 receiving enzyme replacement therapy (ERT). A total of N= 41 patients with AFD were included, of which 26 were receiving ERT.All groups had mean haemoglobin (Hb) within normal reference ranges. Mean soluble transferrin receptor (sTfR) levels were below lab reference ranges (1.8-4.6mg/L) for both ERT and non-ERT receiving patients in GD and AFD groups (GD on ERT mean 1.51mg/L, no ERT 1.47mg.L, AFD on ERT mean 1.04 mg/L, no ERT 0.93mg/L). GD patients had mean ferritin levels elevated above the upper limit of normal reference range (Non-ERT group mean = 453 µg/L, ERT group mean 398 µg/L). For AFD patients, mean ferritin was within in the normal range for both ERT (88 µg/L) and non-ERT (54 µg/L) receiving groups. In all groups, there was a wide range of serum prohepcidin levels (figure 1). Mean prohepcidin was lower in both AFD and GD groups vs controls, but this did not reach statistical significance in any group. There was no correlation between prohepcidin and Hb, serum IL-6 or serum sTfR in any group.
Conclusion
Our study confirms previous results that GD is associated with a hyperferritinaemic state which is not found in AFD. As sTfR levels in GD and AFD patients were low, this appears to be secondary to inflammation rather than iron status. As in previous studies, we found IL-6 levels to be higher in GD patients then controls. Anaemia was not prevalent in any patient groups, although this may be masked by treatment and appropriate transfusion in a carefully monitored patient group. There was no significant difference between prohepcidin levels in AFD or GD patients and controls, and no correlation between Hb, IL-6, or iron status with prohepcidin in any group. The extent to which prohepcidin reflects hepcidin itself is controversial, but there is a growing body of evidence that prohepcidin levels are affected by inflammatory conditions, iron deficiency anaemia and changes in EPO levels, amongst other things. Further studies, with a higher number of patients, and using an ELISA targeted against hepcidin itself are required to further explore what role it may play in AFD/GD patients.

Session topic: E-poster
Keyword(s): Gaucher disease, Iron, Lysosomal storage disease
{{ help_message }}
{{filter}}


