INTERNATIONAL SENTINEL SITE SURVEILLANCE OF PATIENTS WITH TRANSFUSIONAL HEMOSIDEROSIS TREATED WITH DEFERASIROX IN ACTUAL PRACTICE SETTING
(Abstract release date: 05/19/16)
EHA Library. El Beshlawy A. 06/09/16; 133024; E1475

Prof. Dr. Amal El Beshlawy
Contributions
Contributions
Abstract
Abstract: E1475
Type: Eposter Presentation
Background
Long-term iron chelation therapy (ICT) is required in regularly transfused patients (pts) to reduce the chronic iron overload (IOL) which is a major complication causing morbidity and mortality. The oral iron chelator deferasirox (DFX) is indicated for the treatment of chronic IOL due to blood transfusions in adult and pediatric pts aged ≥2 years (yrs). Multiple clinical studies have established the efficacy and safety of DFX in transfusion-dependent pts with IOL. The present study reports the results of a postmarketing active surveillance program for DFX.
Aims
To evaluate the long-term safety and clinical management of DFX in adult and pediatric pts aged ≥2 yrs with chronic transfusional IOL in the actual practice setting.
Methods
Pts aged ≥2 yrs treated with DFX for transfusional hemosiderosis according to the local prescription information were enrolled in this non-interventional study. Data were collected for 3 yrs from initiation of treatment with DFX, and retrospective data were collected in pts who had treatment with DFX for up to 1 yr prior enrollment. The primary endpoints were as follows: (a) the proportion of pts with at least 1 notable increase in serum creatinine (SrCr), defined as >33% above baseline (BL) and the age adjusted upper limit of normal (ULN) in at least 2 consecutive measurements (≥7 days apart), and (b) notable increase in alanine aminotransferase (ALT), defined as >5×ULN in at least 2 consecutive measurements (≥7 days apart).
Results
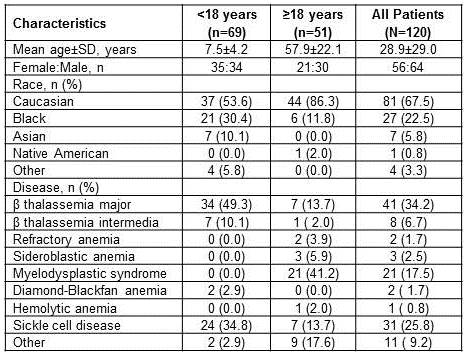
Of the 120 pts enrolled, majority were diagnosed with β thalassemia (n=49), sickle cell disease (SCD, n=31), and myelodysplastic syndrome (MDS, n=21) (Table 1). The mean age (±SD) was 7.5±4.2 yrs in pts with <18 yrs (n=69) and 57.9±22.1 yrs in pts with ≥18 yrs (n=51) of age. Median duration of DFX exposure was 29.9 months and average actual dose was 23.2±8.2 mg/kg/d. Overall, 42.5% (<18 yrs, n=45; ≥18 yrs, n=6) of pts completed the study. The most common reasons for study discontinuation (>10%) were subjects who no longer required study drug (19.2%), adverse events (AEs, 12.5%), and consent withdrawal (10.8%).Dose reductions and interruptions occurred in 39.3% and 23.9% of pts, respectively. Of the 46 pts who had dose reductions, 5 pts reported increase in SrCr (n=3) and ALT (n=2). Of the 28 pts who had dose interruptions, 3 pts reported increase in SrCr (n=2) and ALT (n=1). Notable increase in SrCr was observed in 12% (14 of 117; 95% CI, 7.1-19.2) of pts with MDS (n=3), SCD (n=7) and other anemias (n=4). Notable increase in ALT was observed in 1 pt (0.9%; 95% CI, 0.0-5.2) with β thalassemia (BL ALT missing).Overall, 34.2% (40 of 117) of pts had AEs suspected to be related to DFX, including AEs (>5%) such as diarrhea (9.4%), increase in SrCr (7.7%), and vomiting (6.0%). A total of 4.3% (5 of 117) of pts had serious AEs (SAEs), suspected to be related to DFX, and SAE >1% was gastrointestinal hemorrhage (1.7%). The AEs leading to the discontinuation of DFX occurred in 18.8% of pts (MDS, n=11; β-thalassemia, n=2; SCD, n=2; other anemias, n=7) regardless of the relationship with DFX, including AEs (>2%) such as increase in SrCr (3.4%) and diarrhea (2.6%). None of the pts discontinued DFX due to the increase in ALT.Eight (6.8%; MDS, n=4, other anemias, n=4) on-treatment deaths were reported, all in pts ≥18 yrs of age, majorly due to gastrointestinal disorders (n=2) and neoplasms (n=2). Of these 8 deaths, 7 were not suspected to be related to DFX (data unknown, n=1).
Conclusion
The results demonstrated a safety profile for DFX which is consistent with previously published data. The data demonstrates that changes in SrCr can be managed with dose adjustments or interruptions and occasionally led to permanent discontinuation. DFX has been associated with rare hepatic toxicity. Only 1 pt had a notable increase in ALT. The overall incidence of AEs that lead to DFX discontinuation was higher in pts with MDS during the study.
Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload, Safety
Type: Eposter Presentation
Background
Long-term iron chelation therapy (ICT) is required in regularly transfused patients (pts) to reduce the chronic iron overload (IOL) which is a major complication causing morbidity and mortality. The oral iron chelator deferasirox (DFX) is indicated for the treatment of chronic IOL due to blood transfusions in adult and pediatric pts aged ≥2 years (yrs). Multiple clinical studies have established the efficacy and safety of DFX in transfusion-dependent pts with IOL. The present study reports the results of a postmarketing active surveillance program for DFX.
Aims
To evaluate the long-term safety and clinical management of DFX in adult and pediatric pts aged ≥2 yrs with chronic transfusional IOL in the actual practice setting.
Methods
Pts aged ≥2 yrs treated with DFX for transfusional hemosiderosis according to the local prescription information were enrolled in this non-interventional study. Data were collected for 3 yrs from initiation of treatment with DFX, and retrospective data were collected in pts who had treatment with DFX for up to 1 yr prior enrollment. The primary endpoints were as follows: (a) the proportion of pts with at least 1 notable increase in serum creatinine (SrCr), defined as >33% above baseline (BL) and the age adjusted upper limit of normal (ULN) in at least 2 consecutive measurements (≥7 days apart), and (b) notable increase in alanine aminotransferase (ALT), defined as >5×ULN in at least 2 consecutive measurements (≥7 days apart).
Results
Of the 120 pts enrolled, majority were diagnosed with β thalassemia (n=49), sickle cell disease (SCD, n=31), and myelodysplastic syndrome (MDS, n=21) (Table 1). The mean age (±SD) was 7.5±4.2 yrs in pts with <18 yrs (n=69) and 57.9±22.1 yrs in pts with ≥18 yrs (n=51) of age. Median duration of DFX exposure was 29.9 months and average actual dose was 23.2±8.2 mg/kg/d. Overall, 42.5% (<18 yrs, n=45; ≥18 yrs, n=6) of pts completed the study. The most common reasons for study discontinuation (>10%) were subjects who no longer required study drug (19.2%), adverse events (AEs, 12.5%), and consent withdrawal (10.8%).Dose reductions and interruptions occurred in 39.3% and 23.9% of pts, respectively. Of the 46 pts who had dose reductions, 5 pts reported increase in SrCr (n=3) and ALT (n=2). Of the 28 pts who had dose interruptions, 3 pts reported increase in SrCr (n=2) and ALT (n=1). Notable increase in SrCr was observed in 12% (14 of 117; 95% CI, 7.1-19.2) of pts with MDS (n=3), SCD (n=7) and other anemias (n=4). Notable increase in ALT was observed in 1 pt (0.9%; 95% CI, 0.0-5.2) with β thalassemia (BL ALT missing).Overall, 34.2% (40 of 117) of pts had AEs suspected to be related to DFX, including AEs (>5%) such as diarrhea (9.4%), increase in SrCr (7.7%), and vomiting (6.0%). A total of 4.3% (5 of 117) of pts had serious AEs (SAEs), suspected to be related to DFX, and SAE >1% was gastrointestinal hemorrhage (1.7%). The AEs leading to the discontinuation of DFX occurred in 18.8% of pts (MDS, n=11; β-thalassemia, n=2; SCD, n=2; other anemias, n=7) regardless of the relationship with DFX, including AEs (>2%) such as increase in SrCr (3.4%) and diarrhea (2.6%). None of the pts discontinued DFX due to the increase in ALT.Eight (6.8%; MDS, n=4, other anemias, n=4) on-treatment deaths were reported, all in pts ≥18 yrs of age, majorly due to gastrointestinal disorders (n=2) and neoplasms (n=2). Of these 8 deaths, 7 were not suspected to be related to DFX (data unknown, n=1).
Conclusion
The results demonstrated a safety profile for DFX which is consistent with previously published data. The data demonstrates that changes in SrCr can be managed with dose adjustments or interruptions and occasionally led to permanent discontinuation. DFX has been associated with rare hepatic toxicity. Only 1 pt had a notable increase in ALT. The overall incidence of AEs that lead to DFX discontinuation was higher in pts with MDS during the study.

Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload, Safety
Abstract: E1475
Type: Eposter Presentation
Background
Long-term iron chelation therapy (ICT) is required in regularly transfused patients (pts) to reduce the chronic iron overload (IOL) which is a major complication causing morbidity and mortality. The oral iron chelator deferasirox (DFX) is indicated for the treatment of chronic IOL due to blood transfusions in adult and pediatric pts aged ≥2 years (yrs). Multiple clinical studies have established the efficacy and safety of DFX in transfusion-dependent pts with IOL. The present study reports the results of a postmarketing active surveillance program for DFX.
Aims
To evaluate the long-term safety and clinical management of DFX in adult and pediatric pts aged ≥2 yrs with chronic transfusional IOL in the actual practice setting.
Methods
Pts aged ≥2 yrs treated with DFX for transfusional hemosiderosis according to the local prescription information were enrolled in this non-interventional study. Data were collected for 3 yrs from initiation of treatment with DFX, and retrospective data were collected in pts who had treatment with DFX for up to 1 yr prior enrollment. The primary endpoints were as follows: (a) the proportion of pts with at least 1 notable increase in serum creatinine (SrCr), defined as >33% above baseline (BL) and the age adjusted upper limit of normal (ULN) in at least 2 consecutive measurements (≥7 days apart), and (b) notable increase in alanine aminotransferase (ALT), defined as >5×ULN in at least 2 consecutive measurements (≥7 days apart).
Results
Of the 120 pts enrolled, majority were diagnosed with β thalassemia (n=49), sickle cell disease (SCD, n=31), and myelodysplastic syndrome (MDS, n=21) (Table 1). The mean age (±SD) was 7.5±4.2 yrs in pts with <18 yrs (n=69) and 57.9±22.1 yrs in pts with ≥18 yrs (n=51) of age. Median duration of DFX exposure was 29.9 months and average actual dose was 23.2±8.2 mg/kg/d. Overall, 42.5% (<18 yrs, n=45; ≥18 yrs, n=6) of pts completed the study. The most common reasons for study discontinuation (>10%) were subjects who no longer required study drug (19.2%), adverse events (AEs, 12.5%), and consent withdrawal (10.8%).Dose reductions and interruptions occurred in 39.3% and 23.9% of pts, respectively. Of the 46 pts who had dose reductions, 5 pts reported increase in SrCr (n=3) and ALT (n=2). Of the 28 pts who had dose interruptions, 3 pts reported increase in SrCr (n=2) and ALT (n=1). Notable increase in SrCr was observed in 12% (14 of 117; 95% CI, 7.1-19.2) of pts with MDS (n=3), SCD (n=7) and other anemias (n=4). Notable increase in ALT was observed in 1 pt (0.9%; 95% CI, 0.0-5.2) with β thalassemia (BL ALT missing).Overall, 34.2% (40 of 117) of pts had AEs suspected to be related to DFX, including AEs (>5%) such as diarrhea (9.4%), increase in SrCr (7.7%), and vomiting (6.0%). A total of 4.3% (5 of 117) of pts had serious AEs (SAEs), suspected to be related to DFX, and SAE >1% was gastrointestinal hemorrhage (1.7%). The AEs leading to the discontinuation of DFX occurred in 18.8% of pts (MDS, n=11; β-thalassemia, n=2; SCD, n=2; other anemias, n=7) regardless of the relationship with DFX, including AEs (>2%) such as increase in SrCr (3.4%) and diarrhea (2.6%). None of the pts discontinued DFX due to the increase in ALT.Eight (6.8%; MDS, n=4, other anemias, n=4) on-treatment deaths were reported, all in pts ≥18 yrs of age, majorly due to gastrointestinal disorders (n=2) and neoplasms (n=2). Of these 8 deaths, 7 were not suspected to be related to DFX (data unknown, n=1).
Conclusion
The results demonstrated a safety profile for DFX which is consistent with previously published data. The data demonstrates that changes in SrCr can be managed with dose adjustments or interruptions and occasionally led to permanent discontinuation. DFX has been associated with rare hepatic toxicity. Only 1 pt had a notable increase in ALT. The overall incidence of AEs that lead to DFX discontinuation was higher in pts with MDS during the study.

Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload, Safety
Type: Eposter Presentation
Background
Long-term iron chelation therapy (ICT) is required in regularly transfused patients (pts) to reduce the chronic iron overload (IOL) which is a major complication causing morbidity and mortality. The oral iron chelator deferasirox (DFX) is indicated for the treatment of chronic IOL due to blood transfusions in adult and pediatric pts aged ≥2 years (yrs). Multiple clinical studies have established the efficacy and safety of DFX in transfusion-dependent pts with IOL. The present study reports the results of a postmarketing active surveillance program for DFX.
Aims
To evaluate the long-term safety and clinical management of DFX in adult and pediatric pts aged ≥2 yrs with chronic transfusional IOL in the actual practice setting.
Methods
Pts aged ≥2 yrs treated with DFX for transfusional hemosiderosis according to the local prescription information were enrolled in this non-interventional study. Data were collected for 3 yrs from initiation of treatment with DFX, and retrospective data were collected in pts who had treatment with DFX for up to 1 yr prior enrollment. The primary endpoints were as follows: (a) the proportion of pts with at least 1 notable increase in serum creatinine (SrCr), defined as >33% above baseline (BL) and the age adjusted upper limit of normal (ULN) in at least 2 consecutive measurements (≥7 days apart), and (b) notable increase in alanine aminotransferase (ALT), defined as >5×ULN in at least 2 consecutive measurements (≥7 days apart).
Results
Of the 120 pts enrolled, majority were diagnosed with β thalassemia (n=49), sickle cell disease (SCD, n=31), and myelodysplastic syndrome (MDS, n=21) (Table 1). The mean age (±SD) was 7.5±4.2 yrs in pts with <18 yrs (n=69) and 57.9±22.1 yrs in pts with ≥18 yrs (n=51) of age. Median duration of DFX exposure was 29.9 months and average actual dose was 23.2±8.2 mg/kg/d. Overall, 42.5% (<18 yrs, n=45; ≥18 yrs, n=6) of pts completed the study. The most common reasons for study discontinuation (>10%) were subjects who no longer required study drug (19.2%), adverse events (AEs, 12.5%), and consent withdrawal (10.8%).Dose reductions and interruptions occurred in 39.3% and 23.9% of pts, respectively. Of the 46 pts who had dose reductions, 5 pts reported increase in SrCr (n=3) and ALT (n=2). Of the 28 pts who had dose interruptions, 3 pts reported increase in SrCr (n=2) and ALT (n=1). Notable increase in SrCr was observed in 12% (14 of 117; 95% CI, 7.1-19.2) of pts with MDS (n=3), SCD (n=7) and other anemias (n=4). Notable increase in ALT was observed in 1 pt (0.9%; 95% CI, 0.0-5.2) with β thalassemia (BL ALT missing).Overall, 34.2% (40 of 117) of pts had AEs suspected to be related to DFX, including AEs (>5%) such as diarrhea (9.4%), increase in SrCr (7.7%), and vomiting (6.0%). A total of 4.3% (5 of 117) of pts had serious AEs (SAEs), suspected to be related to DFX, and SAE >1% was gastrointestinal hemorrhage (1.7%). The AEs leading to the discontinuation of DFX occurred in 18.8% of pts (MDS, n=11; β-thalassemia, n=2; SCD, n=2; other anemias, n=7) regardless of the relationship with DFX, including AEs (>2%) such as increase in SrCr (3.4%) and diarrhea (2.6%). None of the pts discontinued DFX due to the increase in ALT.Eight (6.8%; MDS, n=4, other anemias, n=4) on-treatment deaths were reported, all in pts ≥18 yrs of age, majorly due to gastrointestinal disorders (n=2) and neoplasms (n=2). Of these 8 deaths, 7 were not suspected to be related to DFX (data unknown, n=1).
Conclusion
The results demonstrated a safety profile for DFX which is consistent with previously published data. The data demonstrates that changes in SrCr can be managed with dose adjustments or interruptions and occasionally led to permanent discontinuation. DFX has been associated with rare hepatic toxicity. Only 1 pt had a notable increase in ALT. The overall incidence of AEs that lead to DFX discontinuation was higher in pts with MDS during the study.

Session topic: E-poster
Keyword(s): Deferasirox, Iron chelation, Iron overload, Safety
{{ help_message }}
{{filter}}


