TREATMENT WITH CYCLOSPORIN IN AUTO-IMMUNE CYTOPENIAS IN CHILDREN: THE EXPERIENCE FROM THE FRENCH COHORT OBS’CEREVANCE
(Abstract release date: 05/19/16)
EHA Library. Aladjidi N. 06/09/16; 133023; E1474

Dr. Nathalie Aladjidi
Contributions
Contributions
Abstract
Abstract: E1474
Type: Eposter Presentation
Background
Auto-immune cytopenias are rare in children, and there is no recommendation about treatments in case of resistance or dependence to steroids.
Aims
Since 2004, the cohort OBS’CEREVANCE has been recording the cases of auto-immune cytopenias in children treated in the 30 French pediatric hematology units. This study reports the treatment with cyclosporin for all the patients of the cohort treated with cyclosporin for auto-immune hemolytic anemia (AIHA), Immune throbopenic Purpura (ITP) and Evans Syndrome (ES).
Methods
We analyzed the data of 15 AIHA, 7 ITP and 12 ES. Median age was 4.5 years for AIHA and ES, 7.2 for ITP. Ten patients had underlying immunodeficiency and 5 had auto-immune disease.
Results
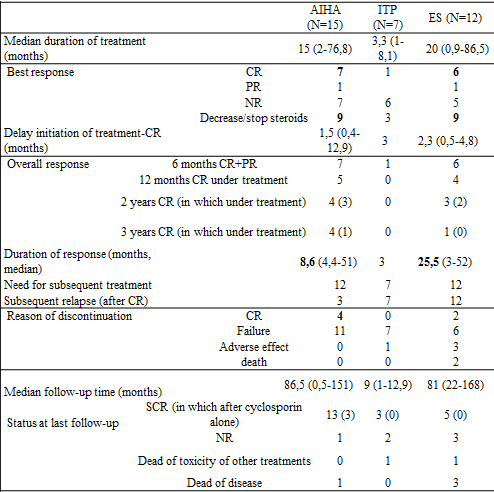
The best response was a complete remission (CR) for 7 AIHA, 1 ITP and 6 ES, maintained in median 8.6 months for AIHA and 25.5 months for ES. The treatment allowed steroids decrease in 9 cases of AIHA and 9 ES. Three patients treated for AIHA maintained CR after discontinuation of treatment, whereas all the patients with ES relapsed during or after treatment. Toxicity was acceptable. Median residual level was 96.5 mg/L (31-369) for patients achieving CR or PR and 117 mg/L (23-956) for patients with no response.
Conclusion
Given our results, we propose to use cyclosporin for AIHA (excluding in context of immunodeficiency) and ES, after failure of corticosteroids, or in case of dependence to steroids. We propose to base the prescription on aplastic anemia treatment recommendations with a blood level residual target between 50 and 100 mg/L: treatment at least three months before concluding to failure, treatment at least a year at full dose in case of response, very slow decrease over several months, return to previous dose in case of relapse during the decrease, or to full doses in case of relapse at treatment discontinuation, monitoring of residuel level, in case of success (CR), toxicity or failure.
Session topic: E-poster
Keyword(s): Autoimmune hemolytic anemia (AIHA), Cyclosporin A, Idiopathic thombocytopenic purpura (ITP), Immunomodulation
Type: Eposter Presentation
Background
Auto-immune cytopenias are rare in children, and there is no recommendation about treatments in case of resistance or dependence to steroids.
Aims
Since 2004, the cohort OBS’CEREVANCE has been recording the cases of auto-immune cytopenias in children treated in the 30 French pediatric hematology units. This study reports the treatment with cyclosporin for all the patients of the cohort treated with cyclosporin for auto-immune hemolytic anemia (AIHA), Immune throbopenic Purpura (ITP) and Evans Syndrome (ES).
Methods
We analyzed the data of 15 AIHA, 7 ITP and 12 ES. Median age was 4.5 years for AIHA and ES, 7.2 for ITP. Ten patients had underlying immunodeficiency and 5 had auto-immune disease.
Results
The best response was a complete remission (CR) for 7 AIHA, 1 ITP and 6 ES, maintained in median 8.6 months for AIHA and 25.5 months for ES. The treatment allowed steroids decrease in 9 cases of AIHA and 9 ES. Three patients treated for AIHA maintained CR after discontinuation of treatment, whereas all the patients with ES relapsed during or after treatment. Toxicity was acceptable. Median residual level was 96.5 mg/L (31-369) for patients achieving CR or PR and 117 mg/L (23-956) for patients with no response.
Conclusion
Given our results, we propose to use cyclosporin for AIHA (excluding in context of immunodeficiency) and ES, after failure of corticosteroids, or in case of dependence to steroids. We propose to base the prescription on aplastic anemia treatment recommendations with a blood level residual target between 50 and 100 mg/L: treatment at least three months before concluding to failure, treatment at least a year at full dose in case of response, very slow decrease over several months, return to previous dose in case of relapse during the decrease, or to full doses in case of relapse at treatment discontinuation, monitoring of residuel level, in case of success (CR), toxicity or failure.

Session topic: E-poster
Keyword(s): Autoimmune hemolytic anemia (AIHA), Cyclosporin A, Idiopathic thombocytopenic purpura (ITP), Immunomodulation
Abstract: E1474
Type: Eposter Presentation
Background
Auto-immune cytopenias are rare in children, and there is no recommendation about treatments in case of resistance or dependence to steroids.
Aims
Since 2004, the cohort OBS’CEREVANCE has been recording the cases of auto-immune cytopenias in children treated in the 30 French pediatric hematology units. This study reports the treatment with cyclosporin for all the patients of the cohort treated with cyclosporin for auto-immune hemolytic anemia (AIHA), Immune throbopenic Purpura (ITP) and Evans Syndrome (ES).
Methods
We analyzed the data of 15 AIHA, 7 ITP and 12 ES. Median age was 4.5 years for AIHA and ES, 7.2 for ITP. Ten patients had underlying immunodeficiency and 5 had auto-immune disease.
Results
The best response was a complete remission (CR) for 7 AIHA, 1 ITP and 6 ES, maintained in median 8.6 months for AIHA and 25.5 months for ES. The treatment allowed steroids decrease in 9 cases of AIHA and 9 ES. Three patients treated for AIHA maintained CR after discontinuation of treatment, whereas all the patients with ES relapsed during or after treatment. Toxicity was acceptable. Median residual level was 96.5 mg/L (31-369) for patients achieving CR or PR and 117 mg/L (23-956) for patients with no response.
Conclusion
Given our results, we propose to use cyclosporin for AIHA (excluding in context of immunodeficiency) and ES, after failure of corticosteroids, or in case of dependence to steroids. We propose to base the prescription on aplastic anemia treatment recommendations with a blood level residual target between 50 and 100 mg/L: treatment at least three months before concluding to failure, treatment at least a year at full dose in case of response, very slow decrease over several months, return to previous dose in case of relapse during the decrease, or to full doses in case of relapse at treatment discontinuation, monitoring of residuel level, in case of success (CR), toxicity or failure.

Session topic: E-poster
Keyword(s): Autoimmune hemolytic anemia (AIHA), Cyclosporin A, Idiopathic thombocytopenic purpura (ITP), Immunomodulation
Type: Eposter Presentation
Background
Auto-immune cytopenias are rare in children, and there is no recommendation about treatments in case of resistance or dependence to steroids.
Aims
Since 2004, the cohort OBS’CEREVANCE has been recording the cases of auto-immune cytopenias in children treated in the 30 French pediatric hematology units. This study reports the treatment with cyclosporin for all the patients of the cohort treated with cyclosporin for auto-immune hemolytic anemia (AIHA), Immune throbopenic Purpura (ITP) and Evans Syndrome (ES).
Methods
We analyzed the data of 15 AIHA, 7 ITP and 12 ES. Median age was 4.5 years for AIHA and ES, 7.2 for ITP. Ten patients had underlying immunodeficiency and 5 had auto-immune disease.
Results
The best response was a complete remission (CR) for 7 AIHA, 1 ITP and 6 ES, maintained in median 8.6 months for AIHA and 25.5 months for ES. The treatment allowed steroids decrease in 9 cases of AIHA and 9 ES. Three patients treated for AIHA maintained CR after discontinuation of treatment, whereas all the patients with ES relapsed during or after treatment. Toxicity was acceptable. Median residual level was 96.5 mg/L (31-369) for patients achieving CR or PR and 117 mg/L (23-956) for patients with no response.
Conclusion
Given our results, we propose to use cyclosporin for AIHA (excluding in context of immunodeficiency) and ES, after failure of corticosteroids, or in case of dependence to steroids. We propose to base the prescription on aplastic anemia treatment recommendations with a blood level residual target between 50 and 100 mg/L: treatment at least three months before concluding to failure, treatment at least a year at full dose in case of response, very slow decrease over several months, return to previous dose in case of relapse during the decrease, or to full doses in case of relapse at treatment discontinuation, monitoring of residuel level, in case of success (CR), toxicity or failure.

Session topic: E-poster
Keyword(s): Autoimmune hemolytic anemia (AIHA), Cyclosporin A, Idiopathic thombocytopenic purpura (ITP), Immunomodulation
{{ help_message }}
{{filter}}


