IGG MEDIATED OPSONIZATION MECHANISM ACCORDING TO THE UNDERLYING PROTEIN DEFICIENCY IN HEREDITARY SPHEROCYTOSIS: TESTING A HYPOTHESIS
(Abstract release date: 05/19/16)
EHA Library. Rocha S. 06/09/16; 133018; E1469

Dr. Susana Rocha
Contributions
Contributions
Abstract
Abstract: E1469
Type: Eposter Presentation
Background
Hereditary Spherocytosis (HS) is the most common non-immune congenital hemolytic anemia in individuals of northern European ancestry, ranging from an asymptomatic condition to a severe life-threatening anemia which results from an erythrocyte membrane protein defect. Splenectomy corrects anemia, though the intrinsic erythrocyte membrane defect persists. Reliene et al. (1) proposed that the type of protein deficiency underlying HS determines erythrocyte in vivo survival since, after splenectomy, the opsonization mechanism mediated by IgG (which involves the binding of natural occurring antibodies to erythrocyte membrane band 3) is distinct for spectrin/ankyrin- and band 3-deficient HS.
Aims
Our study aimed to evaluate the levels of membrane bound IgG and band 3 aggregates, according to the protein deficiency underlying HS, in order to ascertain how our HS population fitted this hypothesis.
Methods
We studied 35 healthy individuals and 125 HS patients [(82 unsplenectomized (unspl) and 43 splenectomized (spl)] previously studied for membrane protein deficiency identification (2). Membrane bound IgG and the high molecular weight aggregates (HMWAg) of band 3 (as percentage of total band 3) were determined by western-blot.
Results
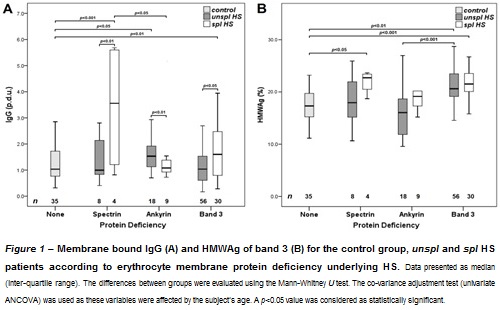
We analyzed our data according to spectrin, ankyrin- and band 3-deficient HS (Figure 1).
Conclusion
For spl band 3-deficient HS, our results were in agreement with the work from Reliene et al. (1), since IgG levels were increased, as well as the amount of band 3 aggregates (to which IgG binds), in comparison with either the control or ankyrin deficiency groups, confirming that in these individuals membrane loss through vesiculation does not include band 3 loss, leaving these cells susceptible to IgG mediated opsonization. Since they found that the amount of IgG was not increased in relation to controls, these same authors (1) proposed that for spl spectrin- and ankyrin-deficient HS, the vesiculation of the membrane leads to the loss of band 3 clusters and, consequently, less opsonization occurred, thus explaining the higher deformability of these patients’ cells. Our results were slightly different because we analyzed spectrin- and ankyrin-deficient HS separately. For spl ankyrin-deficient HS, our findings corroborate those from Reliene et al. (1), as we found lower membrane bound IgG and HMWAg than in band 3 deficiency, and no difference to the control group, meaning that band 3 was lost through vesiculation (hypothesis that is strengthened by HMWAg in unspl ankyrin-deficient being lower than in band 3-deficient HS). However, for spectrin-deficient HS, our results were comparable to those found for band 3 deficiency. This might imply that in spectrin-deficient HS the membrane structural modifications are such that membrane loss does not include band 3, leaving these patients’ cells prone to increased IgG linkage. As cell deformability has not been evaluated, we cannot say if the mechanism of opsonization in spl spectrin-deficient HS is comparable to band 3-deficient or to ankyrin-deficient HS, which is an issue that requires further studies. (1) Reliene et al. Blood 2002,100(6):2208-2215(2) Rocha et al. Br J Haematol 2010, 150(6):679-684. Acknowledgments: This study was sponsored by a PhD grant (SFRH/BPD/80023/2011) attributed to S. Rocha by FCT and FSE and with financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 in association with the reference UCIBIO (UID/MULTI/04378/2013 – POCI/01/0145/FERDER/007728)
Session topic: E-poster
Keyword(s): Band 3, Hereditary spherocytosis, Immunoglobulin, Splenectomy
Type: Eposter Presentation
Background
Hereditary Spherocytosis (HS) is the most common non-immune congenital hemolytic anemia in individuals of northern European ancestry, ranging from an asymptomatic condition to a severe life-threatening anemia which results from an erythrocyte membrane protein defect. Splenectomy corrects anemia, though the intrinsic erythrocyte membrane defect persists. Reliene et al. (1) proposed that the type of protein deficiency underlying HS determines erythrocyte in vivo survival since, after splenectomy, the opsonization mechanism mediated by IgG (which involves the binding of natural occurring antibodies to erythrocyte membrane band 3) is distinct for spectrin/ankyrin- and band 3-deficient HS.
Aims
Our study aimed to evaluate the levels of membrane bound IgG and band 3 aggregates, according to the protein deficiency underlying HS, in order to ascertain how our HS population fitted this hypothesis.
Methods
We studied 35 healthy individuals and 125 HS patients [(82 unsplenectomized (unspl) and 43 splenectomized (spl)] previously studied for membrane protein deficiency identification (2). Membrane bound IgG and the high molecular weight aggregates (HMWAg) of band 3 (as percentage of total band 3) were determined by western-blot.
Results
We analyzed our data according to spectrin, ankyrin- and band 3-deficient HS (Figure 1).
Conclusion
For spl band 3-deficient HS, our results were in agreement with the work from Reliene et al. (1), since IgG levels were increased, as well as the amount of band 3 aggregates (to which IgG binds), in comparison with either the control or ankyrin deficiency groups, confirming that in these individuals membrane loss through vesiculation does not include band 3 loss, leaving these cells susceptible to IgG mediated opsonization. Since they found that the amount of IgG was not increased in relation to controls, these same authors (1) proposed that for spl spectrin- and ankyrin-deficient HS, the vesiculation of the membrane leads to the loss of band 3 clusters and, consequently, less opsonization occurred, thus explaining the higher deformability of these patients’ cells. Our results were slightly different because we analyzed spectrin- and ankyrin-deficient HS separately. For spl ankyrin-deficient HS, our findings corroborate those from Reliene et al. (1), as we found lower membrane bound IgG and HMWAg than in band 3 deficiency, and no difference to the control group, meaning that band 3 was lost through vesiculation (hypothesis that is strengthened by HMWAg in unspl ankyrin-deficient being lower than in band 3-deficient HS). However, for spectrin-deficient HS, our results were comparable to those found for band 3 deficiency. This might imply that in spectrin-deficient HS the membrane structural modifications are such that membrane loss does not include band 3, leaving these patients’ cells prone to increased IgG linkage. As cell deformability has not been evaluated, we cannot say if the mechanism of opsonization in spl spectrin-deficient HS is comparable to band 3-deficient or to ankyrin-deficient HS, which is an issue that requires further studies. (1) Reliene et al. Blood 2002,100(6):2208-2215(2) Rocha et al. Br J Haematol 2010, 150(6):679-684. Acknowledgments: This study was sponsored by a PhD grant (SFRH/BPD/80023/2011) attributed to S. Rocha by FCT and FSE and with financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 in association with the reference UCIBIO (UID/MULTI/04378/2013 – POCI/01/0145/FERDER/007728)

Session topic: E-poster
Keyword(s): Band 3, Hereditary spherocytosis, Immunoglobulin, Splenectomy
Abstract: E1469
Type: Eposter Presentation
Background
Hereditary Spherocytosis (HS) is the most common non-immune congenital hemolytic anemia in individuals of northern European ancestry, ranging from an asymptomatic condition to a severe life-threatening anemia which results from an erythrocyte membrane protein defect. Splenectomy corrects anemia, though the intrinsic erythrocyte membrane defect persists. Reliene et al. (1) proposed that the type of protein deficiency underlying HS determines erythrocyte in vivo survival since, after splenectomy, the opsonization mechanism mediated by IgG (which involves the binding of natural occurring antibodies to erythrocyte membrane band 3) is distinct for spectrin/ankyrin- and band 3-deficient HS.
Aims
Our study aimed to evaluate the levels of membrane bound IgG and band 3 aggregates, according to the protein deficiency underlying HS, in order to ascertain how our HS population fitted this hypothesis.
Methods
We studied 35 healthy individuals and 125 HS patients [(82 unsplenectomized (unspl) and 43 splenectomized (spl)] previously studied for membrane protein deficiency identification (2). Membrane bound IgG and the high molecular weight aggregates (HMWAg) of band 3 (as percentage of total band 3) were determined by western-blot.
Results
We analyzed our data according to spectrin, ankyrin- and band 3-deficient HS (Figure 1).
Conclusion
For spl band 3-deficient HS, our results were in agreement with the work from Reliene et al. (1), since IgG levels were increased, as well as the amount of band 3 aggregates (to which IgG binds), in comparison with either the control or ankyrin deficiency groups, confirming that in these individuals membrane loss through vesiculation does not include band 3 loss, leaving these cells susceptible to IgG mediated opsonization. Since they found that the amount of IgG was not increased in relation to controls, these same authors (1) proposed that for spl spectrin- and ankyrin-deficient HS, the vesiculation of the membrane leads to the loss of band 3 clusters and, consequently, less opsonization occurred, thus explaining the higher deformability of these patients’ cells. Our results were slightly different because we analyzed spectrin- and ankyrin-deficient HS separately. For spl ankyrin-deficient HS, our findings corroborate those from Reliene et al. (1), as we found lower membrane bound IgG and HMWAg than in band 3 deficiency, and no difference to the control group, meaning that band 3 was lost through vesiculation (hypothesis that is strengthened by HMWAg in unspl ankyrin-deficient being lower than in band 3-deficient HS). However, for spectrin-deficient HS, our results were comparable to those found for band 3 deficiency. This might imply that in spectrin-deficient HS the membrane structural modifications are such that membrane loss does not include band 3, leaving these patients’ cells prone to increased IgG linkage. As cell deformability has not been evaluated, we cannot say if the mechanism of opsonization in spl spectrin-deficient HS is comparable to band 3-deficient or to ankyrin-deficient HS, which is an issue that requires further studies. (1) Reliene et al. Blood 2002,100(6):2208-2215(2) Rocha et al. Br J Haematol 2010, 150(6):679-684. Acknowledgments: This study was sponsored by a PhD grant (SFRH/BPD/80023/2011) attributed to S. Rocha by FCT and FSE and with financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 in association with the reference UCIBIO (UID/MULTI/04378/2013 – POCI/01/0145/FERDER/007728)

Session topic: E-poster
Keyword(s): Band 3, Hereditary spherocytosis, Immunoglobulin, Splenectomy
Type: Eposter Presentation
Background
Hereditary Spherocytosis (HS) is the most common non-immune congenital hemolytic anemia in individuals of northern European ancestry, ranging from an asymptomatic condition to a severe life-threatening anemia which results from an erythrocyte membrane protein defect. Splenectomy corrects anemia, though the intrinsic erythrocyte membrane defect persists. Reliene et al. (1) proposed that the type of protein deficiency underlying HS determines erythrocyte in vivo survival since, after splenectomy, the opsonization mechanism mediated by IgG (which involves the binding of natural occurring antibodies to erythrocyte membrane band 3) is distinct for spectrin/ankyrin- and band 3-deficient HS.
Aims
Our study aimed to evaluate the levels of membrane bound IgG and band 3 aggregates, according to the protein deficiency underlying HS, in order to ascertain how our HS population fitted this hypothesis.
Methods
We studied 35 healthy individuals and 125 HS patients [(82 unsplenectomized (unspl) and 43 splenectomized (spl)] previously studied for membrane protein deficiency identification (2). Membrane bound IgG and the high molecular weight aggregates (HMWAg) of band 3 (as percentage of total band 3) were determined by western-blot.
Results
We analyzed our data according to spectrin, ankyrin- and band 3-deficient HS (Figure 1).
Conclusion
For spl band 3-deficient HS, our results were in agreement with the work from Reliene et al. (1), since IgG levels were increased, as well as the amount of band 3 aggregates (to which IgG binds), in comparison with either the control or ankyrin deficiency groups, confirming that in these individuals membrane loss through vesiculation does not include band 3 loss, leaving these cells susceptible to IgG mediated opsonization. Since they found that the amount of IgG was not increased in relation to controls, these same authors (1) proposed that for spl spectrin- and ankyrin-deficient HS, the vesiculation of the membrane leads to the loss of band 3 clusters and, consequently, less opsonization occurred, thus explaining the higher deformability of these patients’ cells. Our results were slightly different because we analyzed spectrin- and ankyrin-deficient HS separately. For spl ankyrin-deficient HS, our findings corroborate those from Reliene et al. (1), as we found lower membrane bound IgG and HMWAg than in band 3 deficiency, and no difference to the control group, meaning that band 3 was lost through vesiculation (hypothesis that is strengthened by HMWAg in unspl ankyrin-deficient being lower than in band 3-deficient HS). However, for spectrin-deficient HS, our results were comparable to those found for band 3 deficiency. This might imply that in spectrin-deficient HS the membrane structural modifications are such that membrane loss does not include band 3, leaving these patients’ cells prone to increased IgG linkage. As cell deformability has not been evaluated, we cannot say if the mechanism of opsonization in spl spectrin-deficient HS is comparable to band 3-deficient or to ankyrin-deficient HS, which is an issue that requires further studies. (1) Reliene et al. Blood 2002,100(6):2208-2215(2) Rocha et al. Br J Haematol 2010, 150(6):679-684. Acknowledgments: This study was sponsored by a PhD grant (SFRH/BPD/80023/2011) attributed to S. Rocha by FCT and FSE and with financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 in association with the reference UCIBIO (UID/MULTI/04378/2013 – POCI/01/0145/FERDER/007728)

Session topic: E-poster
Keyword(s): Band 3, Hereditary spherocytosis, Immunoglobulin, Splenectomy
{{ help_message }}
{{filter}}


