OUT-OF-POCKET ECONOMIC BURDEN AMONG COMMERCIALLY INSURED PATIENTS NEWLY DIAGNOSED WITH MULTIPLE MYELOMA IN THE U.S.
(Abstract release date: 05/19/16)
EHA Library. Seal B. 06/09/16; 133001; E1452

Dr. Brian Seal
Contributions
Contributions
Abstract
Abstract: E1452
Type: Eposter Presentation
Background
Patient (pt) outcomes have markedly improved with the introduction of novel agents for multiple myeloma (MM), but the economic burden of MM management in the U.S. is high. The increasing burden of out-of-pocket (OOP) costs on cancer pts is of concern especially with a shift in insurance coverage towards more cost-sharing. Annual OOP expenditures have been estimated as $5,054 for lung cancer (2015 US $, Romanus 2008) and $5,472 for solid tumors (Zafar 2013). Data on OOP costs in MM in the era of novel treatments are limited.
Aims
This retrospective cohort study estimated overall healthcare costs (OHC) of MM management and OOP burden among commercially insured U.S. pts newly diagnosed with MM (NDMM) in the U.S.
Methods
Pts <65 years of age with NDMM were identified, based on ICD-9-CM codes, in the MarketScan claims database from 1/1/2008 to 9/30/2013. Pts initiating MM treatments were followed from the date of initial MM treatment (index date). All pts had continuous medical and prescription (Rx) commercial insurance coverage 12 months (mos) before (baseline period) and at least 12 mos after (follow-up) the index date. Pts with claims for transplants or Medicare insurance were excluded. Pt socio-demographic characteristics were evaluated during the 12-mo baseline period. Cumulative OHC (sum of payments to the provider paid by the insurance, the patient and other payers) and OOP payments (sum of copayments, co-insurance and deductibles paid by pts) were determined at 1-, 2-, and 3-years post-index date among pts who had continuous commercial insurance coverage through each corresponding year.
Results
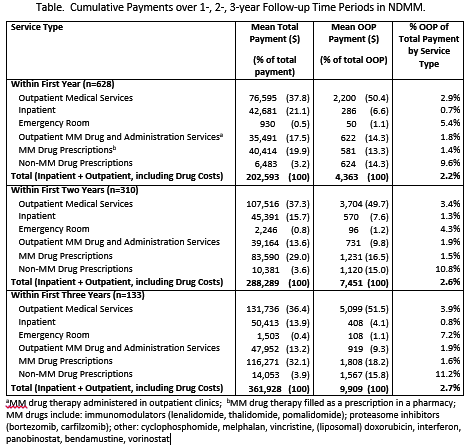
Among 628 commercially insured pts with NDMM, mean age was 56.2 years (SD:6.3); 54.6% were male, 32.8%, 33.4% and 33.8% had a Charlson Comorbidity Index (CCI) score of 0, 1-2, 3+, respectively; 57.2% were enrolled in a preferred provider organization (PPO) plan, 17.7% in a healthcare managed organization (HMO) plan; 38.2% resided in the South, 24.8% in the West, 22.5% in the North Central, and 13.9% in the Northeast regions of the U.S. Mean total healthcare payments per patient in year 1 were $202,593, with $76,595 for outpt medical services, excluding drug and drug administration, $42,681 for inpt services, $35,491 for outpt MM drug and drug administration services, $40,414 for MM drug prescriptions, and $930 for the emergency room (Table). Over time the relative proportions of OHC by service type were consistent. Over the 3 year study period, OOP payments ranged from 2.2% to 2.7% of the total healthcare payments (Table). Total cumulative OOP costs increased from $4,363 in year 1 to $9,909 over the first 3 years after the index date. MM drug related OOP (those administered in outpatient clinics or filled as an Rx) ranged from 1.6% in year 1 and 2 to 1.7% in year 3 of total MM drug treatment expenditures. The main contributor to OOP costs was related to outpatient medical services, $2,200, $3,704 and $5,099 through years 1, 2 and 3, respectively.
Conclusion
In this study based claims data from 2009 to 2013, the main cost driver of OHC and OOP among U.S. NDMM pts receiving treatment was accounted for by non-MM drug therapy related services. Mean MM drug therapy and drug administration related OOP costs were less than $100 per month. These results are based on commercial claims and do not reflect reimbursements from copayment assistance programs.References: Romanus et al. Value in Health 2008. 11:A482. Zafar et al. The Oncologist 2013. 18:381-90.
Session topic: E-poster
Keyword(s): Cost analysis, Multiple myeloma
Type: Eposter Presentation
Background
Patient (pt) outcomes have markedly improved with the introduction of novel agents for multiple myeloma (MM), but the economic burden of MM management in the U.S. is high. The increasing burden of out-of-pocket (OOP) costs on cancer pts is of concern especially with a shift in insurance coverage towards more cost-sharing. Annual OOP expenditures have been estimated as $5,054 for lung cancer (2015 US $, Romanus 2008) and $5,472 for solid tumors (Zafar 2013). Data on OOP costs in MM in the era of novel treatments are limited.
Aims
This retrospective cohort study estimated overall healthcare costs (OHC) of MM management and OOP burden among commercially insured U.S. pts newly diagnosed with MM (NDMM) in the U.S.
Methods
Pts <65 years of age with NDMM were identified, based on ICD-9-CM codes, in the MarketScan claims database from 1/1/2008 to 9/30/2013. Pts initiating MM treatments were followed from the date of initial MM treatment (index date). All pts had continuous medical and prescription (Rx) commercial insurance coverage 12 months (mos) before (baseline period) and at least 12 mos after (follow-up) the index date. Pts with claims for transplants or Medicare insurance were excluded. Pt socio-demographic characteristics were evaluated during the 12-mo baseline period. Cumulative OHC (sum of payments to the provider paid by the insurance, the patient and other payers) and OOP payments (sum of copayments, co-insurance and deductibles paid by pts) were determined at 1-, 2-, and 3-years post-index date among pts who had continuous commercial insurance coverage through each corresponding year.
Results
Among 628 commercially insured pts with NDMM, mean age was 56.2 years (SD:6.3); 54.6% were male, 32.8%, 33.4% and 33.8% had a Charlson Comorbidity Index (CCI) score of 0, 1-2, 3+, respectively; 57.2% were enrolled in a preferred provider organization (PPO) plan, 17.7% in a healthcare managed organization (HMO) plan; 38.2% resided in the South, 24.8% in the West, 22.5% in the North Central, and 13.9% in the Northeast regions of the U.S. Mean total healthcare payments per patient in year 1 were $202,593, with $76,595 for outpt medical services, excluding drug and drug administration, $42,681 for inpt services, $35,491 for outpt MM drug and drug administration services, $40,414 for MM drug prescriptions, and $930 for the emergency room (Table). Over time the relative proportions of OHC by service type were consistent. Over the 3 year study period, OOP payments ranged from 2.2% to 2.7% of the total healthcare payments (Table). Total cumulative OOP costs increased from $4,363 in year 1 to $9,909 over the first 3 years after the index date. MM drug related OOP (those administered in outpatient clinics or filled as an Rx) ranged from 1.6% in year 1 and 2 to 1.7% in year 3 of total MM drug treatment expenditures. The main contributor to OOP costs was related to outpatient medical services, $2,200, $3,704 and $5,099 through years 1, 2 and 3, respectively.
Conclusion
In this study based claims data from 2009 to 2013, the main cost driver of OHC and OOP among U.S. NDMM pts receiving treatment was accounted for by non-MM drug therapy related services. Mean MM drug therapy and drug administration related OOP costs were less than $100 per month. These results are based on commercial claims and do not reflect reimbursements from copayment assistance programs.References: Romanus et al. Value in Health 2008. 11:A482. Zafar et al. The Oncologist 2013. 18:381-90.

Session topic: E-poster
Keyword(s): Cost analysis, Multiple myeloma
Abstract: E1452
Type: Eposter Presentation
Background
Patient (pt) outcomes have markedly improved with the introduction of novel agents for multiple myeloma (MM), but the economic burden of MM management in the U.S. is high. The increasing burden of out-of-pocket (OOP) costs on cancer pts is of concern especially with a shift in insurance coverage towards more cost-sharing. Annual OOP expenditures have been estimated as $5,054 for lung cancer (2015 US $, Romanus 2008) and $5,472 for solid tumors (Zafar 2013). Data on OOP costs in MM in the era of novel treatments are limited.
Aims
This retrospective cohort study estimated overall healthcare costs (OHC) of MM management and OOP burden among commercially insured U.S. pts newly diagnosed with MM (NDMM) in the U.S.
Methods
Pts <65 years of age with NDMM were identified, based on ICD-9-CM codes, in the MarketScan claims database from 1/1/2008 to 9/30/2013. Pts initiating MM treatments were followed from the date of initial MM treatment (index date). All pts had continuous medical and prescription (Rx) commercial insurance coverage 12 months (mos) before (baseline period) and at least 12 mos after (follow-up) the index date. Pts with claims for transplants or Medicare insurance were excluded. Pt socio-demographic characteristics were evaluated during the 12-mo baseline period. Cumulative OHC (sum of payments to the provider paid by the insurance, the patient and other payers) and OOP payments (sum of copayments, co-insurance and deductibles paid by pts) were determined at 1-, 2-, and 3-years post-index date among pts who had continuous commercial insurance coverage through each corresponding year.
Results
Among 628 commercially insured pts with NDMM, mean age was 56.2 years (SD:6.3); 54.6% were male, 32.8%, 33.4% and 33.8% had a Charlson Comorbidity Index (CCI) score of 0, 1-2, 3+, respectively; 57.2% were enrolled in a preferred provider organization (PPO) plan, 17.7% in a healthcare managed organization (HMO) plan; 38.2% resided in the South, 24.8% in the West, 22.5% in the North Central, and 13.9% in the Northeast regions of the U.S. Mean total healthcare payments per patient in year 1 were $202,593, with $76,595 for outpt medical services, excluding drug and drug administration, $42,681 for inpt services, $35,491 for outpt MM drug and drug administration services, $40,414 for MM drug prescriptions, and $930 for the emergency room (Table). Over time the relative proportions of OHC by service type were consistent. Over the 3 year study period, OOP payments ranged from 2.2% to 2.7% of the total healthcare payments (Table). Total cumulative OOP costs increased from $4,363 in year 1 to $9,909 over the first 3 years after the index date. MM drug related OOP (those administered in outpatient clinics or filled as an Rx) ranged from 1.6% in year 1 and 2 to 1.7% in year 3 of total MM drug treatment expenditures. The main contributor to OOP costs was related to outpatient medical services, $2,200, $3,704 and $5,099 through years 1, 2 and 3, respectively.
Conclusion
In this study based claims data from 2009 to 2013, the main cost driver of OHC and OOP among U.S. NDMM pts receiving treatment was accounted for by non-MM drug therapy related services. Mean MM drug therapy and drug administration related OOP costs were less than $100 per month. These results are based on commercial claims and do not reflect reimbursements from copayment assistance programs.References: Romanus et al. Value in Health 2008. 11:A482. Zafar et al. The Oncologist 2013. 18:381-90.

Session topic: E-poster
Keyword(s): Cost analysis, Multiple myeloma
Type: Eposter Presentation
Background
Patient (pt) outcomes have markedly improved with the introduction of novel agents for multiple myeloma (MM), but the economic burden of MM management in the U.S. is high. The increasing burden of out-of-pocket (OOP) costs on cancer pts is of concern especially with a shift in insurance coverage towards more cost-sharing. Annual OOP expenditures have been estimated as $5,054 for lung cancer (2015 US $, Romanus 2008) and $5,472 for solid tumors (Zafar 2013). Data on OOP costs in MM in the era of novel treatments are limited.
Aims
This retrospective cohort study estimated overall healthcare costs (OHC) of MM management and OOP burden among commercially insured U.S. pts newly diagnosed with MM (NDMM) in the U.S.
Methods
Pts <65 years of age with NDMM were identified, based on ICD-9-CM codes, in the MarketScan claims database from 1/1/2008 to 9/30/2013. Pts initiating MM treatments were followed from the date of initial MM treatment (index date). All pts had continuous medical and prescription (Rx) commercial insurance coverage 12 months (mos) before (baseline period) and at least 12 mos after (follow-up) the index date. Pts with claims for transplants or Medicare insurance were excluded. Pt socio-demographic characteristics were evaluated during the 12-mo baseline period. Cumulative OHC (sum of payments to the provider paid by the insurance, the patient and other payers) and OOP payments (sum of copayments, co-insurance and deductibles paid by pts) were determined at 1-, 2-, and 3-years post-index date among pts who had continuous commercial insurance coverage through each corresponding year.
Results
Among 628 commercially insured pts with NDMM, mean age was 56.2 years (SD:6.3); 54.6% were male, 32.8%, 33.4% and 33.8% had a Charlson Comorbidity Index (CCI) score of 0, 1-2, 3+, respectively; 57.2% were enrolled in a preferred provider organization (PPO) plan, 17.7% in a healthcare managed organization (HMO) plan; 38.2% resided in the South, 24.8% in the West, 22.5% in the North Central, and 13.9% in the Northeast regions of the U.S. Mean total healthcare payments per patient in year 1 were $202,593, with $76,595 for outpt medical services, excluding drug and drug administration, $42,681 for inpt services, $35,491 for outpt MM drug and drug administration services, $40,414 for MM drug prescriptions, and $930 for the emergency room (Table). Over time the relative proportions of OHC by service type were consistent. Over the 3 year study period, OOP payments ranged from 2.2% to 2.7% of the total healthcare payments (Table). Total cumulative OOP costs increased from $4,363 in year 1 to $9,909 over the first 3 years after the index date. MM drug related OOP (those administered in outpatient clinics or filled as an Rx) ranged from 1.6% in year 1 and 2 to 1.7% in year 3 of total MM drug treatment expenditures. The main contributor to OOP costs was related to outpatient medical services, $2,200, $3,704 and $5,099 through years 1, 2 and 3, respectively.
Conclusion
In this study based claims data from 2009 to 2013, the main cost driver of OHC and OOP among U.S. NDMM pts receiving treatment was accounted for by non-MM drug therapy related services. Mean MM drug therapy and drug administration related OOP costs were less than $100 per month. These results are based on commercial claims and do not reflect reimbursements from copayment assistance programs.References: Romanus et al. Value in Health 2008. 11:A482. Zafar et al. The Oncologist 2013. 18:381-90.

Session topic: E-poster
Keyword(s): Cost analysis, Multiple myeloma
{{ help_message }}
{{filter}}


