PRIMARY IMMUNE THROMBOCYTOPENIA TREATED WITH ROMIPLOSTIM IN ROUTINE CLINICAL PRACTICE: A RETROSPECTIVE STUDY FROM THE UNITED KINGDOM IMMUNE THROMBOCYTOPENIA REGISTRY
(Abstract release date: 05/19/16)
EHA Library. seesaghur a. 06/09/16; 132975; E1426

Dr. anouchka seesaghur
Contributions
Contributions
Abstract
Abstract: E1426
Type: Eposter Presentation
Background
Immune Thrombocytopenia (ITP) is a rare disorder characterized by low platelet counts, leading to an increased tendency to bleed. Adult chronic ITP patients (pts) in Europe who are refractory to other treatments (e.g. corticosteroids, immunoglobulins) are eligible for treatment with romiplostim, a thrombopoietin-mimetic peptibody. Since ITP treatment-related decisions are principally dependent on clinical expertise or pt preference, observational studies can provide a better understanding of ITP treatment in routine practice.
Aims
To describe the demographic and clinical characteristics of patients with ITP receiving romiplostim in the UK, and to provide details on platelet counts, and the use and pattern of administration of romiplostim in routine clinical practice.
Methods
The United Kingdom Immune Thrombocytopenia (UKITP) Registry retrospectively and prospectively collects demographic and ITP-related clinical data on primary ITP pts enrolled by consent through a network of 68 centres throughout the UK. All adults (≥18yrs) within the UKITP registry who received at least one dose of romiplostim in routine clinical practice from October 2009 until 31st January 2015 were included in the analysis. Data described included demographic and clinical characteristics and ITP medications in patients since ITP diagnosis until end of follow-up and platelet counts before and after romiplostim initiation.
Results
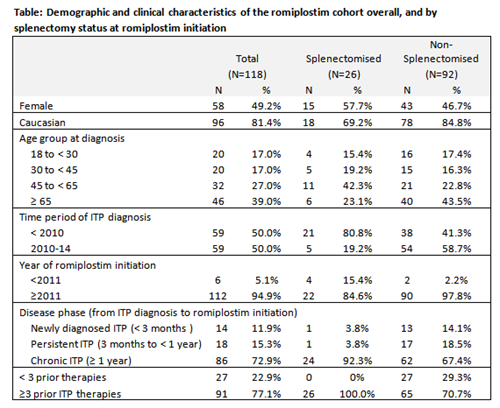
At the time of data extraction from the registry (31st January 2015), a total of 1440 ITP pts were registered and 118 patients were treated with romiplostim. The median age at ITP diagnosis of the romiplostim cohort was 59 years (IQR: 36, 73). Members of the cohort had been diagnosed since 1980, with 50% diagnosed between 2010-14. The median time from ITP diagnosis to romiplostim initiation for those diagnosed between 2010-14 was 0.9 years (IQR: 0.3, 1.9). Almost three-quarters (73%) of patients initiated romiplostim ≥ 1 year from ITP diagnosis, and 12% of patients initiated romiplostim within 3 months of ITP diagnosis. There were some differences in baseline demographics between splenectomised and non-splenectomised patients (Table). Most patients (77%) had received at least three different ITP medications before romiplostim. The most common prior ITP treatments were corticosteroids (90%), intravenous immunoglobulin (IVIg) (77%), and rituximab (57%). The median maximum weekly dose of romiplostim was 3.1 mcg/kg (IQR: 2.0, 6.0). 82% of patients had 2 or fewer ITP medications after romiplostim initiation. The median platelet count within 2 weeks before romiplostim initiation was 17 x 109/L (IQR: 8, 41), which rose to 81 x 109/L (IQR: 31,155) within 1 month of romiplostim treatment and remained >50 x 109/L thereafter.
Conclusion
Limitations include potential selection bias into the registry and the small sample size and heterogeneous nature of the selected cohort. This retrospective analysis of secondary and tertiary care data from the UKITP registry provides a valuable insight in the real-world ITP pt population prescribed with romiplostim in the UK.
Session topic: E-poster
Keyword(s): Chronic ITP, Immune thrombocytopenia (ITP)
Type: Eposter Presentation
Background
Immune Thrombocytopenia (ITP) is a rare disorder characterized by low platelet counts, leading to an increased tendency to bleed. Adult chronic ITP patients (pts) in Europe who are refractory to other treatments (e.g. corticosteroids, immunoglobulins) are eligible for treatment with romiplostim, a thrombopoietin-mimetic peptibody. Since ITP treatment-related decisions are principally dependent on clinical expertise or pt preference, observational studies can provide a better understanding of ITP treatment in routine practice.
Aims
To describe the demographic and clinical characteristics of patients with ITP receiving romiplostim in the UK, and to provide details on platelet counts, and the use and pattern of administration of romiplostim in routine clinical practice.
Methods
The United Kingdom Immune Thrombocytopenia (UKITP) Registry retrospectively and prospectively collects demographic and ITP-related clinical data on primary ITP pts enrolled by consent through a network of 68 centres throughout the UK. All adults (≥18yrs) within the UKITP registry who received at least one dose of romiplostim in routine clinical practice from October 2009 until 31st January 2015 were included in the analysis. Data described included demographic and clinical characteristics and ITP medications in patients since ITP diagnosis until end of follow-up and platelet counts before and after romiplostim initiation.
Results
At the time of data extraction from the registry (31st January 2015), a total of 1440 ITP pts were registered and 118 patients were treated with romiplostim. The median age at ITP diagnosis of the romiplostim cohort was 59 years (IQR: 36, 73). Members of the cohort had been diagnosed since 1980, with 50% diagnosed between 2010-14. The median time from ITP diagnosis to romiplostim initiation for those diagnosed between 2010-14 was 0.9 years (IQR: 0.3, 1.9). Almost three-quarters (73%) of patients initiated romiplostim ≥ 1 year from ITP diagnosis, and 12% of patients initiated romiplostim within 3 months of ITP diagnosis. There were some differences in baseline demographics between splenectomised and non-splenectomised patients (Table). Most patients (77%) had received at least three different ITP medications before romiplostim. The most common prior ITP treatments were corticosteroids (90%), intravenous immunoglobulin (IVIg) (77%), and rituximab (57%). The median maximum weekly dose of romiplostim was 3.1 mcg/kg (IQR: 2.0, 6.0). 82% of patients had 2 or fewer ITP medications after romiplostim initiation. The median platelet count within 2 weeks before romiplostim initiation was 17 x 109/L (IQR: 8, 41), which rose to 81 x 109/L (IQR: 31,155) within 1 month of romiplostim treatment and remained >50 x 109/L thereafter.
Conclusion
Limitations include potential selection bias into the registry and the small sample size and heterogeneous nature of the selected cohort. This retrospective analysis of secondary and tertiary care data from the UKITP registry provides a valuable insight in the real-world ITP pt population prescribed with romiplostim in the UK.

Session topic: E-poster
Keyword(s): Chronic ITP, Immune thrombocytopenia (ITP)
Abstract: E1426
Type: Eposter Presentation
Background
Immune Thrombocytopenia (ITP) is a rare disorder characterized by low platelet counts, leading to an increased tendency to bleed. Adult chronic ITP patients (pts) in Europe who are refractory to other treatments (e.g. corticosteroids, immunoglobulins) are eligible for treatment with romiplostim, a thrombopoietin-mimetic peptibody. Since ITP treatment-related decisions are principally dependent on clinical expertise or pt preference, observational studies can provide a better understanding of ITP treatment in routine practice.
Aims
To describe the demographic and clinical characteristics of patients with ITP receiving romiplostim in the UK, and to provide details on platelet counts, and the use and pattern of administration of romiplostim in routine clinical practice.
Methods
The United Kingdom Immune Thrombocytopenia (UKITP) Registry retrospectively and prospectively collects demographic and ITP-related clinical data on primary ITP pts enrolled by consent through a network of 68 centres throughout the UK. All adults (≥18yrs) within the UKITP registry who received at least one dose of romiplostim in routine clinical practice from October 2009 until 31st January 2015 were included in the analysis. Data described included demographic and clinical characteristics and ITP medications in patients since ITP diagnosis until end of follow-up and platelet counts before and after romiplostim initiation.
Results
At the time of data extraction from the registry (31st January 2015), a total of 1440 ITP pts were registered and 118 patients were treated with romiplostim. The median age at ITP diagnosis of the romiplostim cohort was 59 years (IQR: 36, 73). Members of the cohort had been diagnosed since 1980, with 50% diagnosed between 2010-14. The median time from ITP diagnosis to romiplostim initiation for those diagnosed between 2010-14 was 0.9 years (IQR: 0.3, 1.9). Almost three-quarters (73%) of patients initiated romiplostim ≥ 1 year from ITP diagnosis, and 12% of patients initiated romiplostim within 3 months of ITP diagnosis. There were some differences in baseline demographics between splenectomised and non-splenectomised patients (Table). Most patients (77%) had received at least three different ITP medications before romiplostim. The most common prior ITP treatments were corticosteroids (90%), intravenous immunoglobulin (IVIg) (77%), and rituximab (57%). The median maximum weekly dose of romiplostim was 3.1 mcg/kg (IQR: 2.0, 6.0). 82% of patients had 2 or fewer ITP medications after romiplostim initiation. The median platelet count within 2 weeks before romiplostim initiation was 17 x 109/L (IQR: 8, 41), which rose to 81 x 109/L (IQR: 31,155) within 1 month of romiplostim treatment and remained >50 x 109/L thereafter.
Conclusion
Limitations include potential selection bias into the registry and the small sample size and heterogeneous nature of the selected cohort. This retrospective analysis of secondary and tertiary care data from the UKITP registry provides a valuable insight in the real-world ITP pt population prescribed with romiplostim in the UK.

Session topic: E-poster
Keyword(s): Chronic ITP, Immune thrombocytopenia (ITP)
Type: Eposter Presentation
Background
Immune Thrombocytopenia (ITP) is a rare disorder characterized by low platelet counts, leading to an increased tendency to bleed. Adult chronic ITP patients (pts) in Europe who are refractory to other treatments (e.g. corticosteroids, immunoglobulins) are eligible for treatment with romiplostim, a thrombopoietin-mimetic peptibody. Since ITP treatment-related decisions are principally dependent on clinical expertise or pt preference, observational studies can provide a better understanding of ITP treatment in routine practice.
Aims
To describe the demographic and clinical characteristics of patients with ITP receiving romiplostim in the UK, and to provide details on platelet counts, and the use and pattern of administration of romiplostim in routine clinical practice.
Methods
The United Kingdom Immune Thrombocytopenia (UKITP) Registry retrospectively and prospectively collects demographic and ITP-related clinical data on primary ITP pts enrolled by consent through a network of 68 centres throughout the UK. All adults (≥18yrs) within the UKITP registry who received at least one dose of romiplostim in routine clinical practice from October 2009 until 31st January 2015 were included in the analysis. Data described included demographic and clinical characteristics and ITP medications in patients since ITP diagnosis until end of follow-up and platelet counts before and after romiplostim initiation.
Results
At the time of data extraction from the registry (31st January 2015), a total of 1440 ITP pts were registered and 118 patients were treated with romiplostim. The median age at ITP diagnosis of the romiplostim cohort was 59 years (IQR: 36, 73). Members of the cohort had been diagnosed since 1980, with 50% diagnosed between 2010-14. The median time from ITP diagnosis to romiplostim initiation for those diagnosed between 2010-14 was 0.9 years (IQR: 0.3, 1.9). Almost three-quarters (73%) of patients initiated romiplostim ≥ 1 year from ITP diagnosis, and 12% of patients initiated romiplostim within 3 months of ITP diagnosis. There were some differences in baseline demographics between splenectomised and non-splenectomised patients (Table). Most patients (77%) had received at least three different ITP medications before romiplostim. The most common prior ITP treatments were corticosteroids (90%), intravenous immunoglobulin (IVIg) (77%), and rituximab (57%). The median maximum weekly dose of romiplostim was 3.1 mcg/kg (IQR: 2.0, 6.0). 82% of patients had 2 or fewer ITP medications after romiplostim initiation. The median platelet count within 2 weeks before romiplostim initiation was 17 x 109/L (IQR: 8, 41), which rose to 81 x 109/L (IQR: 31,155) within 1 month of romiplostim treatment and remained >50 x 109/L thereafter.
Conclusion
Limitations include potential selection bias into the registry and the small sample size and heterogeneous nature of the selected cohort. This retrospective analysis of secondary and tertiary care data from the UKITP registry provides a valuable insight in the real-world ITP pt population prescribed with romiplostim in the UK.

Session topic: E-poster
Keyword(s): Chronic ITP, Immune thrombocytopenia (ITP)
{{ help_message }}
{{filter}}


