SAFETY AND EFFICACY OF LONG-TERM OPEN-LABEL DOSING OF SUBCUTANEOUS (SC) ROMIPLOSTIM IN CHILDREN WITH IMMUNE THROMBOCYTOPENIA (ITP)
(Abstract release date: 05/19/16)
EHA Library. Tarantino M. 06/09/16; 132965; E1416
Disclosure(s): Dr. Tarantino is an adviser for Baxalta, Biogen, Grifols, Novo Nordisk, and Pfi zer;
received research funding from Grifols and Novo Nordisk; and is on the
speakers’ bureau with Biogen and Grifols.

Dr. Michael Tarantino
Contributions
Contributions
Abstract
Abstract: E1416
Type: Eposter Presentation
Background
Pediatric patients with chronic immune thrombocytopenia (ITP) that completed the romiplostim phase 1/2 or phase 3 study could enroll in this open‑label long‑term extension study.
Aims
To examine the long-term effects of romiplostim in children with chronic ITP
Methods
All patients received subcutaneous romiplostim once weekly. The initial dose was the final dose from the parent study or 1 µg/kg (for patients previously receiving placebo or that had not received romiplostim for >24 weeks), adjusted weekly from 1–10 µg/kg to target platelet counts of 50‑200×109/L.
Results
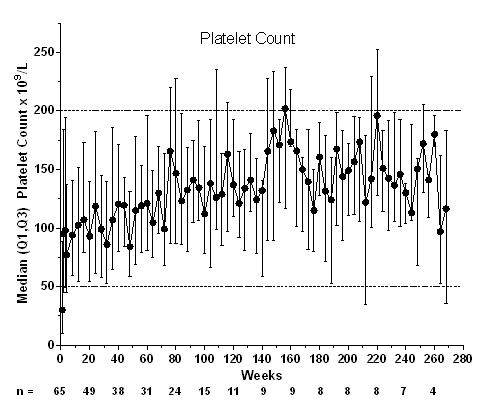
A total of 66 patients (parent study: phase 1/2, n=12; phase 3, n=54) entered the extension study; 1 patient withdrew consent before treatment. At baseline, median (min-max) age was 11 (3–18) years; 56% were female; 61% were white, 14% African American, and 14% Hispanic/Latino; 9.1% had prior splenectomy. Median (min-max) treatment duration was 57.9 (1–269) weeks. Median (range) average weekly romiplostim dose was 5.5 (0.1–10.0) µg/kg. Thirteen patients discontinued treatment: consent withdrawn (n=6), noncompliance (n=2), administrative decision (n=2), nonresponse (n=2), and per protocol (n=1). For 15 patients (23%), the first study week was the first week they were receiving romiplostim (ie, they received placebo in the parent study). Fifty‑six (86%) patients (or caregivers) self‑administered romiplostim. Twenty-one (32%) patients received rescue medications on 63 occasions (for low platelet counts [n=35], bleeding/bruising [17], pre- or post‑procedure [9], and other [2]); treatments included IVIg (n=10), prednisone (9), aminocaproic acid (3), tranexamic acid (2), methylprednisolone (2), and platelet transfusion (1). Patients required rescue treatment during the first 3 months (27/63 instances), >3–6 months (9), >6–9 months (6), >9–12 months (7), and after 1 year (14) in the extension study. Five patients who previously received placebo received rescue medication in this extension, mostly during the first 3 months (10/14 instances). Three patients achieved remission (platelet counts ≥50×109/L for 24 weeks with no ITP treatments): 1) A 9-year-old boy with ITP for 8 years; after 4 years of romiplostim, he entered remission for the last 2 years as of this datacut; 2) An 11-year-old boy with ITP for 6 years; after 3 years of romiplostim, he entered remission for the last year; and 3) A 17-year-old girl with ITP for 8 years; after 6 years of romiplostim, she entered remission for the last 44 weeks of the study. Thirty‑nine serious AEs occurred in 14 patients, including pyrexia (n=3), epistaxis (n=2), and thrombocytopenia (n=2); 3 were deemed treatment-related (anemia, epistaxis, and thrombocytopenia), and none led to discontinuation of romiplostim. Five patients had life‑threatening AEs, including thrombocytopenia (n=2) and infection, decreased platelet counts, and subcutaneous abscess (n=1 each); none were fatal or deemed treatment-related. Bleeding AEs occurred in 47 patients; 3 were deemed treatment-related by the investigator (gingival bleeding, petechiae, and epistaxis). No thrombotic events were reported. There were no peripheral blood abnormalities suggestive of malignancy to warrant a bone marrow examination in any patient. Anti-romiplostim neutralizing antibodies were found in one patient at end of study after 50 weeks. This patient received rescue medications for much of the study.
Conclusion
In this ongoing open-label extension study of children with chronic ITP, romiplostim for ≤5.2 years maintained platelet counts with a safety profile similar to that seen in past studies.
Session topic: E-poster
Keyword(s): Immune thrombocytopenia (ITP), Pediatric, Platelet, Thrombopoietin (TPO)
Type: Eposter Presentation
Background
Pediatric patients with chronic immune thrombocytopenia (ITP) that completed the romiplostim phase 1/2 or phase 3 study could enroll in this open‑label long‑term extension study.
Aims
To examine the long-term effects of romiplostim in children with chronic ITP
Methods
All patients received subcutaneous romiplostim once weekly. The initial dose was the final dose from the parent study or 1 µg/kg (for patients previously receiving placebo or that had not received romiplostim for >24 weeks), adjusted weekly from 1–10 µg/kg to target platelet counts of 50‑200×109/L.
Results
A total of 66 patients (parent study: phase 1/2, n=12; phase 3, n=54) entered the extension study; 1 patient withdrew consent before treatment. At baseline, median (min-max) age was 11 (3–18) years; 56% were female; 61% were white, 14% African American, and 14% Hispanic/Latino; 9.1% had prior splenectomy. Median (min-max) treatment duration was 57.9 (1–269) weeks. Median (range) average weekly romiplostim dose was 5.5 (0.1–10.0) µg/kg. Thirteen patients discontinued treatment: consent withdrawn (n=6), noncompliance (n=2), administrative decision (n=2), nonresponse (n=2), and per protocol (n=1). For 15 patients (23%), the first study week was the first week they were receiving romiplostim (ie, they received placebo in the parent study). Fifty‑six (86%) patients (or caregivers) self‑administered romiplostim. Twenty-one (32%) patients received rescue medications on 63 occasions (for low platelet counts [n=35], bleeding/bruising [17], pre- or post‑procedure [9], and other [2]); treatments included IVIg (n=10), prednisone (9), aminocaproic acid (3), tranexamic acid (2), methylprednisolone (2), and platelet transfusion (1). Patients required rescue treatment during the first 3 months (27/63 instances), >3–6 months (9), >6–9 months (6), >9–12 months (7), and after 1 year (14) in the extension study. Five patients who previously received placebo received rescue medication in this extension, mostly during the first 3 months (10/14 instances). Three patients achieved remission (platelet counts ≥50×109/L for 24 weeks with no ITP treatments): 1) A 9-year-old boy with ITP for 8 years; after 4 years of romiplostim, he entered remission for the last 2 years as of this datacut; 2) An 11-year-old boy with ITP for 6 years; after 3 years of romiplostim, he entered remission for the last year; and 3) A 17-year-old girl with ITP for 8 years; after 6 years of romiplostim, she entered remission for the last 44 weeks of the study. Thirty‑nine serious AEs occurred in 14 patients, including pyrexia (n=3), epistaxis (n=2), and thrombocytopenia (n=2); 3 were deemed treatment-related (anemia, epistaxis, and thrombocytopenia), and none led to discontinuation of romiplostim. Five patients had life‑threatening AEs, including thrombocytopenia (n=2) and infection, decreased platelet counts, and subcutaneous abscess (n=1 each); none were fatal or deemed treatment-related. Bleeding AEs occurred in 47 patients; 3 were deemed treatment-related by the investigator (gingival bleeding, petechiae, and epistaxis). No thrombotic events were reported. There were no peripheral blood abnormalities suggestive of malignancy to warrant a bone marrow examination in any patient. Anti-romiplostim neutralizing antibodies were found in one patient at end of study after 50 weeks. This patient received rescue medications for much of the study.
Conclusion
In this ongoing open-label extension study of children with chronic ITP, romiplostim for ≤5.2 years maintained platelet counts with a safety profile similar to that seen in past studies.

Session topic: E-poster
Keyword(s): Immune thrombocytopenia (ITP), Pediatric, Platelet, Thrombopoietin (TPO)
Abstract: E1416
Type: Eposter Presentation
Background
Pediatric patients with chronic immune thrombocytopenia (ITP) that completed the romiplostim phase 1/2 or phase 3 study could enroll in this open‑label long‑term extension study.
Aims
To examine the long-term effects of romiplostim in children with chronic ITP
Methods
All patients received subcutaneous romiplostim once weekly. The initial dose was the final dose from the parent study or 1 µg/kg (for patients previously receiving placebo or that had not received romiplostim for >24 weeks), adjusted weekly from 1–10 µg/kg to target platelet counts of 50‑200×109/L.
Results
A total of 66 patients (parent study: phase 1/2, n=12; phase 3, n=54) entered the extension study; 1 patient withdrew consent before treatment. At baseline, median (min-max) age was 11 (3–18) years; 56% were female; 61% were white, 14% African American, and 14% Hispanic/Latino; 9.1% had prior splenectomy. Median (min-max) treatment duration was 57.9 (1–269) weeks. Median (range) average weekly romiplostim dose was 5.5 (0.1–10.0) µg/kg. Thirteen patients discontinued treatment: consent withdrawn (n=6), noncompliance (n=2), administrative decision (n=2), nonresponse (n=2), and per protocol (n=1). For 15 patients (23%), the first study week was the first week they were receiving romiplostim (ie, they received placebo in the parent study). Fifty‑six (86%) patients (or caregivers) self‑administered romiplostim. Twenty-one (32%) patients received rescue medications on 63 occasions (for low platelet counts [n=35], bleeding/bruising [17], pre- or post‑procedure [9], and other [2]); treatments included IVIg (n=10), prednisone (9), aminocaproic acid (3), tranexamic acid (2), methylprednisolone (2), and platelet transfusion (1). Patients required rescue treatment during the first 3 months (27/63 instances), >3–6 months (9), >6–9 months (6), >9–12 months (7), and after 1 year (14) in the extension study. Five patients who previously received placebo received rescue medication in this extension, mostly during the first 3 months (10/14 instances). Three patients achieved remission (platelet counts ≥50×109/L for 24 weeks with no ITP treatments): 1) A 9-year-old boy with ITP for 8 years; after 4 years of romiplostim, he entered remission for the last 2 years as of this datacut; 2) An 11-year-old boy with ITP for 6 years; after 3 years of romiplostim, he entered remission for the last year; and 3) A 17-year-old girl with ITP for 8 years; after 6 years of romiplostim, she entered remission for the last 44 weeks of the study. Thirty‑nine serious AEs occurred in 14 patients, including pyrexia (n=3), epistaxis (n=2), and thrombocytopenia (n=2); 3 were deemed treatment-related (anemia, epistaxis, and thrombocytopenia), and none led to discontinuation of romiplostim. Five patients had life‑threatening AEs, including thrombocytopenia (n=2) and infection, decreased platelet counts, and subcutaneous abscess (n=1 each); none were fatal or deemed treatment-related. Bleeding AEs occurred in 47 patients; 3 were deemed treatment-related by the investigator (gingival bleeding, petechiae, and epistaxis). No thrombotic events were reported. There were no peripheral blood abnormalities suggestive of malignancy to warrant a bone marrow examination in any patient. Anti-romiplostim neutralizing antibodies were found in one patient at end of study after 50 weeks. This patient received rescue medications for much of the study.
Conclusion
In this ongoing open-label extension study of children with chronic ITP, romiplostim for ≤5.2 years maintained platelet counts with a safety profile similar to that seen in past studies.

Session topic: E-poster
Keyword(s): Immune thrombocytopenia (ITP), Pediatric, Platelet, Thrombopoietin (TPO)
Type: Eposter Presentation
Background
Pediatric patients with chronic immune thrombocytopenia (ITP) that completed the romiplostim phase 1/2 or phase 3 study could enroll in this open‑label long‑term extension study.
Aims
To examine the long-term effects of romiplostim in children with chronic ITP
Methods
All patients received subcutaneous romiplostim once weekly. The initial dose was the final dose from the parent study or 1 µg/kg (for patients previously receiving placebo or that had not received romiplostim for >24 weeks), adjusted weekly from 1–10 µg/kg to target platelet counts of 50‑200×109/L.
Results
A total of 66 patients (parent study: phase 1/2, n=12; phase 3, n=54) entered the extension study; 1 patient withdrew consent before treatment. At baseline, median (min-max) age was 11 (3–18) years; 56% were female; 61% were white, 14% African American, and 14% Hispanic/Latino; 9.1% had prior splenectomy. Median (min-max) treatment duration was 57.9 (1–269) weeks. Median (range) average weekly romiplostim dose was 5.5 (0.1–10.0) µg/kg. Thirteen patients discontinued treatment: consent withdrawn (n=6), noncompliance (n=2), administrative decision (n=2), nonresponse (n=2), and per protocol (n=1). For 15 patients (23%), the first study week was the first week they were receiving romiplostim (ie, they received placebo in the parent study). Fifty‑six (86%) patients (or caregivers) self‑administered romiplostim. Twenty-one (32%) patients received rescue medications on 63 occasions (for low platelet counts [n=35], bleeding/bruising [17], pre- or post‑procedure [9], and other [2]); treatments included IVIg (n=10), prednisone (9), aminocaproic acid (3), tranexamic acid (2), methylprednisolone (2), and platelet transfusion (1). Patients required rescue treatment during the first 3 months (27/63 instances), >3–6 months (9), >6–9 months (6), >9–12 months (7), and after 1 year (14) in the extension study. Five patients who previously received placebo received rescue medication in this extension, mostly during the first 3 months (10/14 instances). Three patients achieved remission (platelet counts ≥50×109/L for 24 weeks with no ITP treatments): 1) A 9-year-old boy with ITP for 8 years; after 4 years of romiplostim, he entered remission for the last 2 years as of this datacut; 2) An 11-year-old boy with ITP for 6 years; after 3 years of romiplostim, he entered remission for the last year; and 3) A 17-year-old girl with ITP for 8 years; after 6 years of romiplostim, she entered remission for the last 44 weeks of the study. Thirty‑nine serious AEs occurred in 14 patients, including pyrexia (n=3), epistaxis (n=2), and thrombocytopenia (n=2); 3 were deemed treatment-related (anemia, epistaxis, and thrombocytopenia), and none led to discontinuation of romiplostim. Five patients had life‑threatening AEs, including thrombocytopenia (n=2) and infection, decreased platelet counts, and subcutaneous abscess (n=1 each); none were fatal or deemed treatment-related. Bleeding AEs occurred in 47 patients; 3 were deemed treatment-related by the investigator (gingival bleeding, petechiae, and epistaxis). No thrombotic events were reported. There were no peripheral blood abnormalities suggestive of malignancy to warrant a bone marrow examination in any patient. Anti-romiplostim neutralizing antibodies were found in one patient at end of study after 50 weeks. This patient received rescue medications for much of the study.
Conclusion
In this ongoing open-label extension study of children with chronic ITP, romiplostim for ≤5.2 years maintained platelet counts with a safety profile similar to that seen in past studies.

Session topic: E-poster
Keyword(s): Immune thrombocytopenia (ITP), Pediatric, Platelet, Thrombopoietin (TPO)
{{ help_message }}
{{filter}}


