DECREASED PLATELET FUNCTION IN ACUTE MYELOID LEUKAEMIA AND MYELODYSPLASIA DETECTED BY A NEW TEST OF PLATELET AGGREGATION OF VIABLE CELLS USING FLOW CYTOMETRY
(Abstract release date: 05/19/16)
EHA Library. Just Vinholt P. 06/09/16; 132963; E1414

Dr. Pernille Just Vinholt
Contributions
Contributions
Abstract
Abstract: E1414
Type: Eposter Presentation
Background
It is unknown why only some thrombocytopenic patients bleed despite equally low platelet counts. Platelet function is likely to be of importance, but is difficult to access as results from existing platelet aggregation methods have major methodological limitations at low platelet count.
Aims
The aim was to establish a method for measuring platelet aggregation of viable platelets in thrombocytopenic patients. Secondly, we investigated platelet function in thrombocytopenic patients with acute myeloid leukaemia (AML) or myelodysplasia (MDS).
Methods
Washed platelets were split in two and differently labelled with fluorescent dye (Calcein AM and Calcein Violet) and added to AB-positive donor plasma in a 1.5 mL tube. Agonist was added (collagen-related peptide, adenosine diphosphate (ADP) or thrombin-receptor activator peptide-6 (TRAP)), samples were shaken for 5 min and subsequently analyzed using flow cytometry. Double-colored events in stimulated samples were defined as platelet aggregates. Effect of addition of platelet inhibitors (abciximab, ticagrelor or vorapaxar) was determined. Using flow cytometry, platelet activation after addition of agonist to whole blood was evaluated by fl ow cytometry using antibodies to detect alpha-granule release (P-selectin(CD62p)), dense-granule release (CD63) and activated fibrinogen receptor (PAC1 binding), respectively. Surface receptor levels were quantified using antibodies against GPIIb/IIIa (CD41/CD61), GPIb(CD42b)/GPIX(CD42a)), and CD49b. Participants were ≥18 years, healthy individuals or patients with AML or MDS. Participants who received platelet inhibitors within two weeks, platelet transfusions, or major surgery within one week were excluded. Informed consent was obtained. Comparisons were made with Wilcoxon ranksum test.
Results
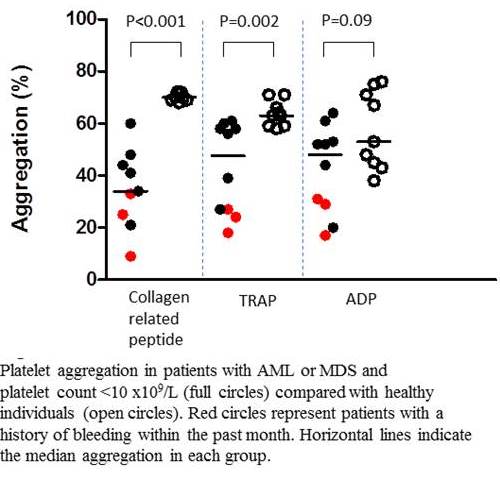
Presence of aggregates in unstimulated samples was low (≤4%). The intra-assay coefficient of variation was 1-3%. Increasing inhibition of platelet aggregation at increasing dose of platelet inhibitors was observed. Enrolled were ten patients (n=8 AML/n=2 MDS) and nine healthy controls with platelet count ranging 6-47 x 109/L versus 145-337 x 109/L, respectively. The inter-individual variation among healthy persons were low (Figure 1). Collagen-related peptide and TRAP-induced aggregation were lower for patients compared with controls, while no difference in ADP-induced aggregation was found. Three patients reported bleeding episodes within the past one month and comprised three out of four persons with platelet aggregation below 34% for any agonist. No difference was observed in platelet count in patients with versus without bleeding episodes: 23 x109/L (12-24 x109/L) versus 25 x109/L (6-47 x109/L), p=0.51. Platelet activation was lower for patients versus controls for all agonists. There was a strong correlation between results from the platelet activation method and platelet aggregation method e.g. for PAC1-binding versus collagen-related peptide induced aggregation, r=0.96, p<0.001. No difference in expression of platelet surface receptor levels was found.
Conclusion
Based on the presented method for testing platelet aggregation of viable cells, our study shows that low platelet aggregation by the method identifies thrombocytopenic patients with bleeding problems among AML/MDS patients with thrombocytopenia. This platelet aggregation method may be useful for providing indications for prophylactic platelet transfusions or for evaluating indication of platelet inhibitor therapy in thrombocytopenic individuals. Further, it may facilitate the important distinction between inherited thrombocytopenia from immune thrombocytopenia.
Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Bleeding, Flow cytometry, Platelet aggregation
Type: Eposter Presentation
Background
It is unknown why only some thrombocytopenic patients bleed despite equally low platelet counts. Platelet function is likely to be of importance, but is difficult to access as results from existing platelet aggregation methods have major methodological limitations at low platelet count.
Aims
The aim was to establish a method for measuring platelet aggregation of viable platelets in thrombocytopenic patients. Secondly, we investigated platelet function in thrombocytopenic patients with acute myeloid leukaemia (AML) or myelodysplasia (MDS).
Methods
Washed platelets were split in two and differently labelled with fluorescent dye (Calcein AM and Calcein Violet) and added to AB-positive donor plasma in a 1.5 mL tube. Agonist was added (collagen-related peptide, adenosine diphosphate (ADP) or thrombin-receptor activator peptide-6 (TRAP)), samples were shaken for 5 min and subsequently analyzed using flow cytometry. Double-colored events in stimulated samples were defined as platelet aggregates. Effect of addition of platelet inhibitors (abciximab, ticagrelor or vorapaxar) was determined. Using flow cytometry, platelet activation after addition of agonist to whole blood was evaluated by fl ow cytometry using antibodies to detect alpha-granule release (P-selectin(CD62p)), dense-granule release (CD63) and activated fibrinogen receptor (PAC1 binding), respectively. Surface receptor levels were quantified using antibodies against GPIIb/IIIa (CD41/CD61), GPIb(CD42b)/GPIX(CD42a)), and CD49b. Participants were ≥18 years, healthy individuals or patients with AML or MDS. Participants who received platelet inhibitors within two weeks, platelet transfusions, or major surgery within one week were excluded. Informed consent was obtained. Comparisons were made with Wilcoxon ranksum test.
Results
Presence of aggregates in unstimulated samples was low (≤4%). The intra-assay coefficient of variation was 1-3%. Increasing inhibition of platelet aggregation at increasing dose of platelet inhibitors was observed. Enrolled were ten patients (n=8 AML/n=2 MDS) and nine healthy controls with platelet count ranging 6-47 x 109/L versus 145-337 x 109/L, respectively. The inter-individual variation among healthy persons were low (Figure 1). Collagen-related peptide and TRAP-induced aggregation were lower for patients compared with controls, while no difference in ADP-induced aggregation was found. Three patients reported bleeding episodes within the past one month and comprised three out of four persons with platelet aggregation below 34% for any agonist. No difference was observed in platelet count in patients with versus without bleeding episodes: 23 x109/L (12-24 x109/L) versus 25 x109/L (6-47 x109/L), p=0.51. Platelet activation was lower for patients versus controls for all agonists. There was a strong correlation between results from the platelet activation method and platelet aggregation method e.g. for PAC1-binding versus collagen-related peptide induced aggregation, r=0.96, p<0.001. No difference in expression of platelet surface receptor levels was found.
Conclusion
Based on the presented method for testing platelet aggregation of viable cells, our study shows that low platelet aggregation by the method identifies thrombocytopenic patients with bleeding problems among AML/MDS patients with thrombocytopenia. This platelet aggregation method may be useful for providing indications for prophylactic platelet transfusions or for evaluating indication of platelet inhibitor therapy in thrombocytopenic individuals. Further, it may facilitate the important distinction between inherited thrombocytopenia from immune thrombocytopenia.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Bleeding, Flow cytometry, Platelet aggregation
Abstract: E1414
Type: Eposter Presentation
Background
It is unknown why only some thrombocytopenic patients bleed despite equally low platelet counts. Platelet function is likely to be of importance, but is difficult to access as results from existing platelet aggregation methods have major methodological limitations at low platelet count.
Aims
The aim was to establish a method for measuring platelet aggregation of viable platelets in thrombocytopenic patients. Secondly, we investigated platelet function in thrombocytopenic patients with acute myeloid leukaemia (AML) or myelodysplasia (MDS).
Methods
Washed platelets were split in two and differently labelled with fluorescent dye (Calcein AM and Calcein Violet) and added to AB-positive donor plasma in a 1.5 mL tube. Agonist was added (collagen-related peptide, adenosine diphosphate (ADP) or thrombin-receptor activator peptide-6 (TRAP)), samples were shaken for 5 min and subsequently analyzed using flow cytometry. Double-colored events in stimulated samples were defined as platelet aggregates. Effect of addition of platelet inhibitors (abciximab, ticagrelor or vorapaxar) was determined. Using flow cytometry, platelet activation after addition of agonist to whole blood was evaluated by fl ow cytometry using antibodies to detect alpha-granule release (P-selectin(CD62p)), dense-granule release (CD63) and activated fibrinogen receptor (PAC1 binding), respectively. Surface receptor levels were quantified using antibodies against GPIIb/IIIa (CD41/CD61), GPIb(CD42b)/GPIX(CD42a)), and CD49b. Participants were ≥18 years, healthy individuals or patients with AML or MDS. Participants who received platelet inhibitors within two weeks, platelet transfusions, or major surgery within one week were excluded. Informed consent was obtained. Comparisons were made with Wilcoxon ranksum test.
Results
Presence of aggregates in unstimulated samples was low (≤4%). The intra-assay coefficient of variation was 1-3%. Increasing inhibition of platelet aggregation at increasing dose of platelet inhibitors was observed. Enrolled were ten patients (n=8 AML/n=2 MDS) and nine healthy controls with platelet count ranging 6-47 x 109/L versus 145-337 x 109/L, respectively. The inter-individual variation among healthy persons were low (Figure 1). Collagen-related peptide and TRAP-induced aggregation were lower for patients compared with controls, while no difference in ADP-induced aggregation was found. Three patients reported bleeding episodes within the past one month and comprised three out of four persons with platelet aggregation below 34% for any agonist. No difference was observed in platelet count in patients with versus without bleeding episodes: 23 x109/L (12-24 x109/L) versus 25 x109/L (6-47 x109/L), p=0.51. Platelet activation was lower for patients versus controls for all agonists. There was a strong correlation between results from the platelet activation method and platelet aggregation method e.g. for PAC1-binding versus collagen-related peptide induced aggregation, r=0.96, p<0.001. No difference in expression of platelet surface receptor levels was found.
Conclusion
Based on the presented method for testing platelet aggregation of viable cells, our study shows that low platelet aggregation by the method identifies thrombocytopenic patients with bleeding problems among AML/MDS patients with thrombocytopenia. This platelet aggregation method may be useful for providing indications for prophylactic platelet transfusions or for evaluating indication of platelet inhibitor therapy in thrombocytopenic individuals. Further, it may facilitate the important distinction between inherited thrombocytopenia from immune thrombocytopenia.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Bleeding, Flow cytometry, Platelet aggregation
Type: Eposter Presentation
Background
It is unknown why only some thrombocytopenic patients bleed despite equally low platelet counts. Platelet function is likely to be of importance, but is difficult to access as results from existing platelet aggregation methods have major methodological limitations at low platelet count.
Aims
The aim was to establish a method for measuring platelet aggregation of viable platelets in thrombocytopenic patients. Secondly, we investigated platelet function in thrombocytopenic patients with acute myeloid leukaemia (AML) or myelodysplasia (MDS).
Methods
Washed platelets were split in two and differently labelled with fluorescent dye (Calcein AM and Calcein Violet) and added to AB-positive donor plasma in a 1.5 mL tube. Agonist was added (collagen-related peptide, adenosine diphosphate (ADP) or thrombin-receptor activator peptide-6 (TRAP)), samples were shaken for 5 min and subsequently analyzed using flow cytometry. Double-colored events in stimulated samples were defined as platelet aggregates. Effect of addition of platelet inhibitors (abciximab, ticagrelor or vorapaxar) was determined. Using flow cytometry, platelet activation after addition of agonist to whole blood was evaluated by fl ow cytometry using antibodies to detect alpha-granule release (P-selectin(CD62p)), dense-granule release (CD63) and activated fibrinogen receptor (PAC1 binding), respectively. Surface receptor levels were quantified using antibodies against GPIIb/IIIa (CD41/CD61), GPIb(CD42b)/GPIX(CD42a)), and CD49b. Participants were ≥18 years, healthy individuals or patients with AML or MDS. Participants who received platelet inhibitors within two weeks, platelet transfusions, or major surgery within one week were excluded. Informed consent was obtained. Comparisons were made with Wilcoxon ranksum test.
Results
Presence of aggregates in unstimulated samples was low (≤4%). The intra-assay coefficient of variation was 1-3%. Increasing inhibition of platelet aggregation at increasing dose of platelet inhibitors was observed. Enrolled were ten patients (n=8 AML/n=2 MDS) and nine healthy controls with platelet count ranging 6-47 x 109/L versus 145-337 x 109/L, respectively. The inter-individual variation among healthy persons were low (Figure 1). Collagen-related peptide and TRAP-induced aggregation were lower for patients compared with controls, while no difference in ADP-induced aggregation was found. Three patients reported bleeding episodes within the past one month and comprised three out of four persons with platelet aggregation below 34% for any agonist. No difference was observed in platelet count in patients with versus without bleeding episodes: 23 x109/L (12-24 x109/L) versus 25 x109/L (6-47 x109/L), p=0.51. Platelet activation was lower for patients versus controls for all agonists. There was a strong correlation between results from the platelet activation method and platelet aggregation method e.g. for PAC1-binding versus collagen-related peptide induced aggregation, r=0.96, p<0.001. No difference in expression of platelet surface receptor levels was found.
Conclusion
Based on the presented method for testing platelet aggregation of viable cells, our study shows that low platelet aggregation by the method identifies thrombocytopenic patients with bleeding problems among AML/MDS patients with thrombocytopenia. This platelet aggregation method may be useful for providing indications for prophylactic platelet transfusions or for evaluating indication of platelet inhibitor therapy in thrombocytopenic individuals. Further, it may facilitate the important distinction between inherited thrombocytopenia from immune thrombocytopenia.

Session topic: E-poster
Keyword(s): Acute myeloid leukemia, Bleeding, Flow cytometry, Platelet aggregation
{{ help_message }}
{{filter}}


