POINT OF CARE PLATELET REACTIVITY TESTING AND DOSE-TITRATION IN A DOUBLE-BLIND STUDY OF PRASUGREL IN CHILDREN WITH SICKLE CELL ANAEMIA
(Abstract release date: 05/19/16)
EHA Library. A Jakubowski J. 06/09/16; 132961; E1412

Dr. Joseph A Jakubowski
Contributions
Contributions
Abstract
Abstract: E1412
Type: Eposter Presentation
Background
Platelet activation and aggregation induced by release of ADP from haemolysed sickle erythrocytes may contribute to vaso-occlusion and ischaemic pain, a hallmark of sickle cell anaemia (SCA). Anti-platelet agents such as prasugrel (Pras) that target the P2Y12 ADP receptor may thus provide benefit. However, the potential increase in haemorrhagic stroke risk with high levels of platelet inhibition in SCA makes dose titration imperative. Previous studies suggested that a PRU (P2Y12 reaction unit, a measure of ADP-induced platelet aggregation) range of 231-136 would be appropriate to determine the risk / benefit ratio of prasugrel in SCA, and could be achieved by individual dose adjustment based on platelet testing - a challenge in a double blind study.
Aims
Attenuation of platelet reactivity to a pre-defined target range in patients with SCA, by dose titration using point-of-care platelet testing.
Methods
The DOVE study was a Phase 3 double-blind, placebo (Pbo) controlled study of Pras in pediatric patients with SCA. Treatment assignment was balanced according to hydroxyurea (HU) use, age group and country. Baseline PRU values were determined by VerifyNow® P2Y12 (VN-P2Y12), a point-of-care platelet aggregation test which reports both PRU and % platelet inhibition (% inhibition) values. Over the subsequent 6 weeks patients returned for repeat VN-P2Y12 assessment and, if necessary doses were adjusted to titrate patients into a PRU range of 231-136, with a lower PRU value indicating greater platelet inhibition. The final dose was fixed for the remainder of the study, adjusted only if deemed necessary by changes in body weight. The majority of patients returned after 9 months for a final VN-P2Y12 measurement. All VN-P2Y12 data were encrypted and entered into an interactive voice response system which instructed the investigator to maintain or adjust the current dose for patients in both the Pras and Pbo (mock titration) arms, thus maintaining the blinding of the study.
Results
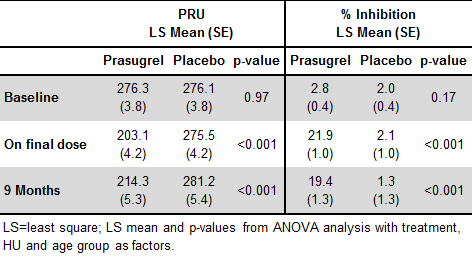
170 patients were assigned to the treatment arm and received Pras; of these, 160 were titrated to a final dose. Baseline (predose) PRU values were similar for both Pras and Pbo groups (Table). After titration to the final Pras dose, PRU values dropped to 203 (from 276 at baseline) and remained low through 9 months. In contrast and as expected, PRU values in the Pbo group did not change after mock titration or at 9 months. Baseline VNP2Y12-reported % inhibition levels were low in both Pras (2.8%) and Pbo (2%) groups. Mean % inhibition in the Pras group increased to approximately 22% after titration to the final dose and was maintained at this approximate level (19.4%) through 9 months of treatment. In the Pbo group, there was no change from baseline in % inhibition at either timepoint.
Conclusion
Although primary efficacy endpoints were not met in the DOVE study, the results of platelet function studies indicate that in the majority of patients, Pras at the final dose administered effectively reduced ADP-mediated platelet reactivity with a corresponding increase in platelet inhibition. This was achieved using encrypted point-of-care testing. Additionally, results demonstrated that safety was not compromised on the treatment arm, raising the question as to whether higher levels of platelet inhibition might provide a more optimal benefit/risk ratio.
Session topic: E-poster
Keyword(s): Anti-platelet therapies, Platelet reactivity, Point-of-care, Sickle cell anemia
Type: Eposter Presentation
Background
Platelet activation and aggregation induced by release of ADP from haemolysed sickle erythrocytes may contribute to vaso-occlusion and ischaemic pain, a hallmark of sickle cell anaemia (SCA). Anti-platelet agents such as prasugrel (Pras) that target the P2Y12 ADP receptor may thus provide benefit. However, the potential increase in haemorrhagic stroke risk with high levels of platelet inhibition in SCA makes dose titration imperative. Previous studies suggested that a PRU (P2Y12 reaction unit, a measure of ADP-induced platelet aggregation) range of 231-136 would be appropriate to determine the risk / benefit ratio of prasugrel in SCA, and could be achieved by individual dose adjustment based on platelet testing - a challenge in a double blind study.
Aims
Attenuation of platelet reactivity to a pre-defined target range in patients with SCA, by dose titration using point-of-care platelet testing.
Methods
The DOVE study was a Phase 3 double-blind, placebo (Pbo) controlled study of Pras in pediatric patients with SCA. Treatment assignment was balanced according to hydroxyurea (HU) use, age group and country. Baseline PRU values were determined by VerifyNow® P2Y12 (VN-P2Y12), a point-of-care platelet aggregation test which reports both PRU and % platelet inhibition (% inhibition) values. Over the subsequent 6 weeks patients returned for repeat VN-P2Y12 assessment and, if necessary doses were adjusted to titrate patients into a PRU range of 231-136, with a lower PRU value indicating greater platelet inhibition. The final dose was fixed for the remainder of the study, adjusted only if deemed necessary by changes in body weight. The majority of patients returned after 9 months for a final VN-P2Y12 measurement. All VN-P2Y12 data were encrypted and entered into an interactive voice response system which instructed the investigator to maintain or adjust the current dose for patients in both the Pras and Pbo (mock titration) arms, thus maintaining the blinding of the study.
Results
170 patients were assigned to the treatment arm and received Pras; of these, 160 were titrated to a final dose. Baseline (predose) PRU values were similar for both Pras and Pbo groups (Table). After titration to the final Pras dose, PRU values dropped to 203 (from 276 at baseline) and remained low through 9 months. In contrast and as expected, PRU values in the Pbo group did not change after mock titration or at 9 months. Baseline VNP2Y12-reported % inhibition levels were low in both Pras (2.8%) and Pbo (2%) groups. Mean % inhibition in the Pras group increased to approximately 22% after titration to the final dose and was maintained at this approximate level (19.4%) through 9 months of treatment. In the Pbo group, there was no change from baseline in % inhibition at either timepoint.
Conclusion
Although primary efficacy endpoints were not met in the DOVE study, the results of platelet function studies indicate that in the majority of patients, Pras at the final dose administered effectively reduced ADP-mediated platelet reactivity with a corresponding increase in platelet inhibition. This was achieved using encrypted point-of-care testing. Additionally, results demonstrated that safety was not compromised on the treatment arm, raising the question as to whether higher levels of platelet inhibition might provide a more optimal benefit/risk ratio.

Session topic: E-poster
Keyword(s): Anti-platelet therapies, Platelet reactivity, Point-of-care, Sickle cell anemia
Abstract: E1412
Type: Eposter Presentation
Background
Platelet activation and aggregation induced by release of ADP from haemolysed sickle erythrocytes may contribute to vaso-occlusion and ischaemic pain, a hallmark of sickle cell anaemia (SCA). Anti-platelet agents such as prasugrel (Pras) that target the P2Y12 ADP receptor may thus provide benefit. However, the potential increase in haemorrhagic stroke risk with high levels of platelet inhibition in SCA makes dose titration imperative. Previous studies suggested that a PRU (P2Y12 reaction unit, a measure of ADP-induced platelet aggregation) range of 231-136 would be appropriate to determine the risk / benefit ratio of prasugrel in SCA, and could be achieved by individual dose adjustment based on platelet testing - a challenge in a double blind study.
Aims
Attenuation of platelet reactivity to a pre-defined target range in patients with SCA, by dose titration using point-of-care platelet testing.
Methods
The DOVE study was a Phase 3 double-blind, placebo (Pbo) controlled study of Pras in pediatric patients with SCA. Treatment assignment was balanced according to hydroxyurea (HU) use, age group and country. Baseline PRU values were determined by VerifyNow® P2Y12 (VN-P2Y12), a point-of-care platelet aggregation test which reports both PRU and % platelet inhibition (% inhibition) values. Over the subsequent 6 weeks patients returned for repeat VN-P2Y12 assessment and, if necessary doses were adjusted to titrate patients into a PRU range of 231-136, with a lower PRU value indicating greater platelet inhibition. The final dose was fixed for the remainder of the study, adjusted only if deemed necessary by changes in body weight. The majority of patients returned after 9 months for a final VN-P2Y12 measurement. All VN-P2Y12 data were encrypted and entered into an interactive voice response system which instructed the investigator to maintain or adjust the current dose for patients in both the Pras and Pbo (mock titration) arms, thus maintaining the blinding of the study.
Results
170 patients were assigned to the treatment arm and received Pras; of these, 160 were titrated to a final dose. Baseline (predose) PRU values were similar for both Pras and Pbo groups (Table). After titration to the final Pras dose, PRU values dropped to 203 (from 276 at baseline) and remained low through 9 months. In contrast and as expected, PRU values in the Pbo group did not change after mock titration or at 9 months. Baseline VNP2Y12-reported % inhibition levels were low in both Pras (2.8%) and Pbo (2%) groups. Mean % inhibition in the Pras group increased to approximately 22% after titration to the final dose and was maintained at this approximate level (19.4%) through 9 months of treatment. In the Pbo group, there was no change from baseline in % inhibition at either timepoint.
Conclusion
Although primary efficacy endpoints were not met in the DOVE study, the results of platelet function studies indicate that in the majority of patients, Pras at the final dose administered effectively reduced ADP-mediated platelet reactivity with a corresponding increase in platelet inhibition. This was achieved using encrypted point-of-care testing. Additionally, results demonstrated that safety was not compromised on the treatment arm, raising the question as to whether higher levels of platelet inhibition might provide a more optimal benefit/risk ratio.

Session topic: E-poster
Keyword(s): Anti-platelet therapies, Platelet reactivity, Point-of-care, Sickle cell anemia
Type: Eposter Presentation
Background
Platelet activation and aggregation induced by release of ADP from haemolysed sickle erythrocytes may contribute to vaso-occlusion and ischaemic pain, a hallmark of sickle cell anaemia (SCA). Anti-platelet agents such as prasugrel (Pras) that target the P2Y12 ADP receptor may thus provide benefit. However, the potential increase in haemorrhagic stroke risk with high levels of platelet inhibition in SCA makes dose titration imperative. Previous studies suggested that a PRU (P2Y12 reaction unit, a measure of ADP-induced platelet aggregation) range of 231-136 would be appropriate to determine the risk / benefit ratio of prasugrel in SCA, and could be achieved by individual dose adjustment based on platelet testing - a challenge in a double blind study.
Aims
Attenuation of platelet reactivity to a pre-defined target range in patients with SCA, by dose titration using point-of-care platelet testing.
Methods
The DOVE study was a Phase 3 double-blind, placebo (Pbo) controlled study of Pras in pediatric patients with SCA. Treatment assignment was balanced according to hydroxyurea (HU) use, age group and country. Baseline PRU values were determined by VerifyNow® P2Y12 (VN-P2Y12), a point-of-care platelet aggregation test which reports both PRU and % platelet inhibition (% inhibition) values. Over the subsequent 6 weeks patients returned for repeat VN-P2Y12 assessment and, if necessary doses were adjusted to titrate patients into a PRU range of 231-136, with a lower PRU value indicating greater platelet inhibition. The final dose was fixed for the remainder of the study, adjusted only if deemed necessary by changes in body weight. The majority of patients returned after 9 months for a final VN-P2Y12 measurement. All VN-P2Y12 data were encrypted and entered into an interactive voice response system which instructed the investigator to maintain or adjust the current dose for patients in both the Pras and Pbo (mock titration) arms, thus maintaining the blinding of the study.
Results
170 patients were assigned to the treatment arm and received Pras; of these, 160 were titrated to a final dose. Baseline (predose) PRU values were similar for both Pras and Pbo groups (Table). After titration to the final Pras dose, PRU values dropped to 203 (from 276 at baseline) and remained low through 9 months. In contrast and as expected, PRU values in the Pbo group did not change after mock titration or at 9 months. Baseline VNP2Y12-reported % inhibition levels were low in both Pras (2.8%) and Pbo (2%) groups. Mean % inhibition in the Pras group increased to approximately 22% after titration to the final dose and was maintained at this approximate level (19.4%) through 9 months of treatment. In the Pbo group, there was no change from baseline in % inhibition at either timepoint.
Conclusion
Although primary efficacy endpoints were not met in the DOVE study, the results of platelet function studies indicate that in the majority of patients, Pras at the final dose administered effectively reduced ADP-mediated platelet reactivity with a corresponding increase in platelet inhibition. This was achieved using encrypted point-of-care testing. Additionally, results demonstrated that safety was not compromised on the treatment arm, raising the question as to whether higher levels of platelet inhibition might provide a more optimal benefit/risk ratio.

Session topic: E-poster
Keyword(s): Anti-platelet therapies, Platelet reactivity, Point-of-care, Sickle cell anemia
{{ help_message }}
{{filter}}


