HYPERFERRITINEMIA AND SERUM INFLAMMATORY CYTOKINES IN ADULTS WITH GAUCHER DISEASE TYPE 1
(Abstract release date: 05/19/16)
EHA Library. Machaczka M. 06/09/16; 132952; E1403

Prof. Dr. Maciej Machaczka
Contributions
Contributions
Abstract
Abstract: E1403
Type: Eposter Presentation
Background
Gaucher disease type 1 (GD1, OMIM #230800) is a rare multisystem lysosomal storage disorder (autosomal recessive). The storage of glucosylceramide in macrophages produces an inflammatory response with iron recycling dysregulation and a release of cytokines. Patients with GD1 suffer from an increased susceptibility to malignancies (e.g., multiple myeloma), and while the underlying mechanisms are not known, it is postulated to be associated with macrophage dysfunction and immune dysregulation.
Aims
This study was undertaken to evaluate ferritinemia, iron metabolism profiles and inflammatory cytokine concentrations in Swedish patients with GD1.
Methods
The study included 16 adults with GD1 aged 20–86 years. All but one patient (94%) carried at least one allele with c.1226A>G (N370S) mutation in the GBA1 gene. Zimran’s severity score index (SSI) was calculated for all patients at the time of their inclusion in the study. The following laboratory variables were collected from fresh blood for analysis: iron profile (s-ferritin, p-iron, p-TSAT); HFE gene mutations; s-hepcidin; LFTs; CRP; serum IL-1β, IL-6, IL-8, IL-10, TNF-α; serum sIL-2Rα. Assessment of the aforementioned variables was performed at baseline (the first blood sampling at Karolinska) and at follow-up (every 6–12 months).
Results
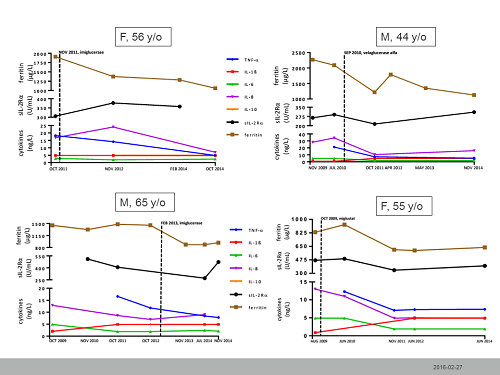
Hyperferritinemia >500 µg/L was present in all but 3 patients (81%). Values of P-transferrin, P-Fe, and P-TSAT were within normal limits for all but one patient. There was no correlation between hyperferritinemia and patient’s sex, spleen status, and clinical status as defined by the Zimran’s SSI. HFE gene mutations were analyzed in 11 patients: 4 pts were heterozygous for His63Asp mutation, one pt was heterozygous for both His63Asp and Cys282Tyr mutations, and 6 patients had no mutation in the HFE gene. No obvious correlation between ferritinemia and HFE genotype was detected in the studied group. Serum hepcidin concentrations were analyzed in 10 patients; 7 pts had normal hepcidin results; one pt with a normal ferritinemia (75 µg/L) had a low hepcidin concentration at 4 µg/L and 2 pts with a hyperferritinemia of 1910 and 833 µg/L had elevated hepcidin at 50 and 55 µg/L, respectively.Cytokine and inflammatory marker analysisThe serum concentrations of IL-1β, IL-8, IL-10 were normal in all studied GD1 patients. The mean and median serum concentrations of TNF-α, IL-6, sIL-2Rα, and CRP were within normal limits in all studied patients. However, in 5 of 11 patients (45%) TNF-α was moderately increased. Additionally, 2 patients with the highest TNF-α concentrations (28.4, and 30.4 ng/L) showed mildly elevated IL-6 concentrations (5.9 and 10.7 ng/L, respectively). In the first aforementioned patient, CRP was increased to 12 mg/L but was normal in the second patient. Of note, the first patient was diagnosed with concomitant chronic lung infection (Mycobacterium avium and Aspergillus fumigatus). Serum levels of IL-6 were within normal limits in the remaining 14 patients (87%). Serum sIL-2Rα concentrations were within normal range in 62% of patients (10/16).Longitudinal assessment of hyperferritinemia and inflammatory cytokines in treated patients with GD1Eight patients with at least 36 months’ follow-up data were eligible for a longitudinal assessment of ferritinemia, TNF-α, IL-6, IL-8, and sIL-2Rα. Four patients were untreated when their baseline sampling was performed and began GD1 therapy. All remained on unchanged therapy throughout follow-up. Longitudinal assessments of the studied variables for the treated patients are presented in the figure.
Conclusion
The study revealed that hyperferritinemia is common in Swedish GD1 patients. Unlike hemophagocytic lymphohistiocytosis, hyperferritinemia in GD1 is not associated with high serum levels of sIL-2Rα. In some, but not all patients TNF-α and IL-6 levels could be mildly elevated. GD1 therapy has a potential to improve ferritinemia and cytokine levels.
Session topic: E-poster
Keyword(s): Cytokine, Ferritin, Gaucher disease
Type: Eposter Presentation
Background
Gaucher disease type 1 (GD1, OMIM #230800) is a rare multisystem lysosomal storage disorder (autosomal recessive). The storage of glucosylceramide in macrophages produces an inflammatory response with iron recycling dysregulation and a release of cytokines. Patients with GD1 suffer from an increased susceptibility to malignancies (e.g., multiple myeloma), and while the underlying mechanisms are not known, it is postulated to be associated with macrophage dysfunction and immune dysregulation.
Aims
This study was undertaken to evaluate ferritinemia, iron metabolism profiles and inflammatory cytokine concentrations in Swedish patients with GD1.
Methods
The study included 16 adults with GD1 aged 20–86 years. All but one patient (94%) carried at least one allele with c.1226A>G (N370S) mutation in the GBA1 gene. Zimran’s severity score index (SSI) was calculated for all patients at the time of their inclusion in the study. The following laboratory variables were collected from fresh blood for analysis: iron profile (s-ferritin, p-iron, p-TSAT); HFE gene mutations; s-hepcidin; LFTs; CRP; serum IL-1β, IL-6, IL-8, IL-10, TNF-α; serum sIL-2Rα. Assessment of the aforementioned variables was performed at baseline (the first blood sampling at Karolinska) and at follow-up (every 6–12 months).
Results
Hyperferritinemia >500 µg/L was present in all but 3 patients (81%). Values of P-transferrin, P-Fe, and P-TSAT were within normal limits for all but one patient. There was no correlation between hyperferritinemia and patient’s sex, spleen status, and clinical status as defined by the Zimran’s SSI. HFE gene mutations were analyzed in 11 patients: 4 pts were heterozygous for His63Asp mutation, one pt was heterozygous for both His63Asp and Cys282Tyr mutations, and 6 patients had no mutation in the HFE gene. No obvious correlation between ferritinemia and HFE genotype was detected in the studied group. Serum hepcidin concentrations were analyzed in 10 patients; 7 pts had normal hepcidin results; one pt with a normal ferritinemia (75 µg/L) had a low hepcidin concentration at 4 µg/L and 2 pts with a hyperferritinemia of 1910 and 833 µg/L had elevated hepcidin at 50 and 55 µg/L, respectively.Cytokine and inflammatory marker analysisThe serum concentrations of IL-1β, IL-8, IL-10 were normal in all studied GD1 patients. The mean and median serum concentrations of TNF-α, IL-6, sIL-2Rα, and CRP were within normal limits in all studied patients. However, in 5 of 11 patients (45%) TNF-α was moderately increased. Additionally, 2 patients with the highest TNF-α concentrations (28.4, and 30.4 ng/L) showed mildly elevated IL-6 concentrations (5.9 and 10.7 ng/L, respectively). In the first aforementioned patient, CRP was increased to 12 mg/L but was normal in the second patient. Of note, the first patient was diagnosed with concomitant chronic lung infection (Mycobacterium avium and Aspergillus fumigatus). Serum levels of IL-6 were within normal limits in the remaining 14 patients (87%). Serum sIL-2Rα concentrations were within normal range in 62% of patients (10/16).Longitudinal assessment of hyperferritinemia and inflammatory cytokines in treated patients with GD1Eight patients with at least 36 months’ follow-up data were eligible for a longitudinal assessment of ferritinemia, TNF-α, IL-6, IL-8, and sIL-2Rα. Four patients were untreated when their baseline sampling was performed and began GD1 therapy. All remained on unchanged therapy throughout follow-up. Longitudinal assessments of the studied variables for the treated patients are presented in the figure.
Conclusion
The study revealed that hyperferritinemia is common in Swedish GD1 patients. Unlike hemophagocytic lymphohistiocytosis, hyperferritinemia in GD1 is not associated with high serum levels of sIL-2Rα. In some, but not all patients TNF-α and IL-6 levels could be mildly elevated. GD1 therapy has a potential to improve ferritinemia and cytokine levels.

Session topic: E-poster
Keyword(s): Cytokine, Ferritin, Gaucher disease
Abstract: E1403
Type: Eposter Presentation
Background
Gaucher disease type 1 (GD1, OMIM #230800) is a rare multisystem lysosomal storage disorder (autosomal recessive). The storage of glucosylceramide in macrophages produces an inflammatory response with iron recycling dysregulation and a release of cytokines. Patients with GD1 suffer from an increased susceptibility to malignancies (e.g., multiple myeloma), and while the underlying mechanisms are not known, it is postulated to be associated with macrophage dysfunction and immune dysregulation.
Aims
This study was undertaken to evaluate ferritinemia, iron metabolism profiles and inflammatory cytokine concentrations in Swedish patients with GD1.
Methods
The study included 16 adults with GD1 aged 20–86 years. All but one patient (94%) carried at least one allele with c.1226A>G (N370S) mutation in the GBA1 gene. Zimran’s severity score index (SSI) was calculated for all patients at the time of their inclusion in the study. The following laboratory variables were collected from fresh blood for analysis: iron profile (s-ferritin, p-iron, p-TSAT); HFE gene mutations; s-hepcidin; LFTs; CRP; serum IL-1β, IL-6, IL-8, IL-10, TNF-α; serum sIL-2Rα. Assessment of the aforementioned variables was performed at baseline (the first blood sampling at Karolinska) and at follow-up (every 6–12 months).
Results
Hyperferritinemia >500 µg/L was present in all but 3 patients (81%). Values of P-transferrin, P-Fe, and P-TSAT were within normal limits for all but one patient. There was no correlation between hyperferritinemia and patient’s sex, spleen status, and clinical status as defined by the Zimran’s SSI. HFE gene mutations were analyzed in 11 patients: 4 pts were heterozygous for His63Asp mutation, one pt was heterozygous for both His63Asp and Cys282Tyr mutations, and 6 patients had no mutation in the HFE gene. No obvious correlation between ferritinemia and HFE genotype was detected in the studied group. Serum hepcidin concentrations were analyzed in 10 patients; 7 pts had normal hepcidin results; one pt with a normal ferritinemia (75 µg/L) had a low hepcidin concentration at 4 µg/L and 2 pts with a hyperferritinemia of 1910 and 833 µg/L had elevated hepcidin at 50 and 55 µg/L, respectively.Cytokine and inflammatory marker analysisThe serum concentrations of IL-1β, IL-8, IL-10 were normal in all studied GD1 patients. The mean and median serum concentrations of TNF-α, IL-6, sIL-2Rα, and CRP were within normal limits in all studied patients. However, in 5 of 11 patients (45%) TNF-α was moderately increased. Additionally, 2 patients with the highest TNF-α concentrations (28.4, and 30.4 ng/L) showed mildly elevated IL-6 concentrations (5.9 and 10.7 ng/L, respectively). In the first aforementioned patient, CRP was increased to 12 mg/L but was normal in the second patient. Of note, the first patient was diagnosed with concomitant chronic lung infection (Mycobacterium avium and Aspergillus fumigatus). Serum levels of IL-6 were within normal limits in the remaining 14 patients (87%). Serum sIL-2Rα concentrations were within normal range in 62% of patients (10/16).Longitudinal assessment of hyperferritinemia and inflammatory cytokines in treated patients with GD1Eight patients with at least 36 months’ follow-up data were eligible for a longitudinal assessment of ferritinemia, TNF-α, IL-6, IL-8, and sIL-2Rα. Four patients were untreated when their baseline sampling was performed and began GD1 therapy. All remained on unchanged therapy throughout follow-up. Longitudinal assessments of the studied variables for the treated patients are presented in the figure.
Conclusion
The study revealed that hyperferritinemia is common in Swedish GD1 patients. Unlike hemophagocytic lymphohistiocytosis, hyperferritinemia in GD1 is not associated with high serum levels of sIL-2Rα. In some, but not all patients TNF-α and IL-6 levels could be mildly elevated. GD1 therapy has a potential to improve ferritinemia and cytokine levels.

Session topic: E-poster
Keyword(s): Cytokine, Ferritin, Gaucher disease
Type: Eposter Presentation
Background
Gaucher disease type 1 (GD1, OMIM #230800) is a rare multisystem lysosomal storage disorder (autosomal recessive). The storage of glucosylceramide in macrophages produces an inflammatory response with iron recycling dysregulation and a release of cytokines. Patients with GD1 suffer from an increased susceptibility to malignancies (e.g., multiple myeloma), and while the underlying mechanisms are not known, it is postulated to be associated with macrophage dysfunction and immune dysregulation.
Aims
This study was undertaken to evaluate ferritinemia, iron metabolism profiles and inflammatory cytokine concentrations in Swedish patients with GD1.
Methods
The study included 16 adults with GD1 aged 20–86 years. All but one patient (94%) carried at least one allele with c.1226A>G (N370S) mutation in the GBA1 gene. Zimran’s severity score index (SSI) was calculated for all patients at the time of their inclusion in the study. The following laboratory variables were collected from fresh blood for analysis: iron profile (s-ferritin, p-iron, p-TSAT); HFE gene mutations; s-hepcidin; LFTs; CRP; serum IL-1β, IL-6, IL-8, IL-10, TNF-α; serum sIL-2Rα. Assessment of the aforementioned variables was performed at baseline (the first blood sampling at Karolinska) and at follow-up (every 6–12 months).
Results
Hyperferritinemia >500 µg/L was present in all but 3 patients (81%). Values of P-transferrin, P-Fe, and P-TSAT were within normal limits for all but one patient. There was no correlation between hyperferritinemia and patient’s sex, spleen status, and clinical status as defined by the Zimran’s SSI. HFE gene mutations were analyzed in 11 patients: 4 pts were heterozygous for His63Asp mutation, one pt was heterozygous for both His63Asp and Cys282Tyr mutations, and 6 patients had no mutation in the HFE gene. No obvious correlation between ferritinemia and HFE genotype was detected in the studied group. Serum hepcidin concentrations were analyzed in 10 patients; 7 pts had normal hepcidin results; one pt with a normal ferritinemia (75 µg/L) had a low hepcidin concentration at 4 µg/L and 2 pts with a hyperferritinemia of 1910 and 833 µg/L had elevated hepcidin at 50 and 55 µg/L, respectively.Cytokine and inflammatory marker analysisThe serum concentrations of IL-1β, IL-8, IL-10 were normal in all studied GD1 patients. The mean and median serum concentrations of TNF-α, IL-6, sIL-2Rα, and CRP were within normal limits in all studied patients. However, in 5 of 11 patients (45%) TNF-α was moderately increased. Additionally, 2 patients with the highest TNF-α concentrations (28.4, and 30.4 ng/L) showed mildly elevated IL-6 concentrations (5.9 and 10.7 ng/L, respectively). In the first aforementioned patient, CRP was increased to 12 mg/L but was normal in the second patient. Of note, the first patient was diagnosed with concomitant chronic lung infection (Mycobacterium avium and Aspergillus fumigatus). Serum levels of IL-6 were within normal limits in the remaining 14 patients (87%). Serum sIL-2Rα concentrations were within normal range in 62% of patients (10/16).Longitudinal assessment of hyperferritinemia and inflammatory cytokines in treated patients with GD1Eight patients with at least 36 months’ follow-up data were eligible for a longitudinal assessment of ferritinemia, TNF-α, IL-6, IL-8, and sIL-2Rα. Four patients were untreated when their baseline sampling was performed and began GD1 therapy. All remained on unchanged therapy throughout follow-up. Longitudinal assessments of the studied variables for the treated patients are presented in the figure.
Conclusion
The study revealed that hyperferritinemia is common in Swedish GD1 patients. Unlike hemophagocytic lymphohistiocytosis, hyperferritinemia in GD1 is not associated with high serum levels of sIL-2Rα. In some, but not all patients TNF-α and IL-6 levels could be mildly elevated. GD1 therapy has a potential to improve ferritinemia and cytokine levels.

Session topic: E-poster
Keyword(s): Cytokine, Ferritin, Gaucher disease
{{ help_message }}
{{filter}}


