ROLE OF BORTEZOMIB IN COMBINATION WITH AEROBIC OXIDATION INHIBITOR ON BURKITT LYMPHOMA CELLS
(Abstract release date: 05/19/16)
EHA Library. Wang T. 06/09/16; 132947; E1398

Prof. Dr. Ting Wang
Contributions
Contributions
Abstract
Abstract: E1398
Type: Eposter Presentation
Background
Caner and non-malignant cells vary vitally with respect to their metabolic pathway. Consuming elevated levels of glucose from a frequently nutrient-poor environment in order to satiate anabolic respiratory reactions, is a sufficiently prevalent phenomenon of cancer cell metabolic adaptions, which has been conceptualized as the “Warburg effect”. However, the molecular mechanism underlying this metabolic reprogramming is obscure. Recent investigations propose that the oncogene-directed metabolic reprogramming, rather than the permanent malfunction of mitochondrial oxidative phosphorylation (OXPHOS), may have profound effects on aerobic glycolysis. The biochemical aspects of the Warburg effect outline a strong explanation for the cause of cancer cell proliferation. However, the more reliant of malignant cells manifest on glycolysis, the more vulnerable to drugs targeting this pathway they will be. Prognosis of Burkitt lymphoma, the most aggressive B-cell lymphoma, is poor even in the Rituximab era. Therefore, targeting the aberrant metabolic pathway rather than illimitably increase the dose of chemotherapy prompts a highly desirable strategy for treating this aggressive lymphoma. Bortezomib is the first proteasome inhibitor approved for treating multiple myeloma and mantel cell lymphoma. In addition to the well-known mechanism on proteasome inhibition, bortezomib is supposed to have effects on the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway, which is deemed crucial to underpin the glycolysis of malignant cells. Given the central role of glycolysis in cancer metabolism, we sought to explore the effect of bortezomib on tumor cell metabolism in Burkitt lymphoma. Considering the fact that even counteracting glycolysis, cancer cells shows low-glucose resistance, which may associate with an increased compensatory upregulation of mitochondrial OXPHOS, the present study was undertaken to investigate the potential of using Bortezomib in combination with OXPHOS inhibitor as a novel therapy for Burkitt lymphoma.
Aims
To investigate the effect of bortezomib (BTZ) in combination with aerobic oxidation inhibitor oligomycin (OM) on proliferation and apoptosis of Burkitt lymphoma cell line Raji, and to explore its possible molecular mechanism.
Methods
Raji cells were treated with different concentrations of BTZ (0, 5, 10, 15, 20, 25 and 30 nmol/L) alone or in combination with OM (0.05 µg/mL). Cell proliferation was detected by CCK-8 method. The mRNA and protein expression levels of oncogene C-myc, hypoxia inducible factor-1α (HIF-1α) and its target genes vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT1), as well as key enzymes and proteins related to glycolysis pathway including hexokinase II (HKII), lactic dehydrogenase (LDHA) and succinate dehydrogenase (SDHA) were detected by real-time fluorescent quantitative PCR and Western blotting, respectively. Glucose consumption and lactic acid generation were examined by Glucose (hexokinase, HK) Assay Kit and Lactate Assay Kit, respectively. Apoptosis and cell cycle distribution were analyzed by FCM.
Results
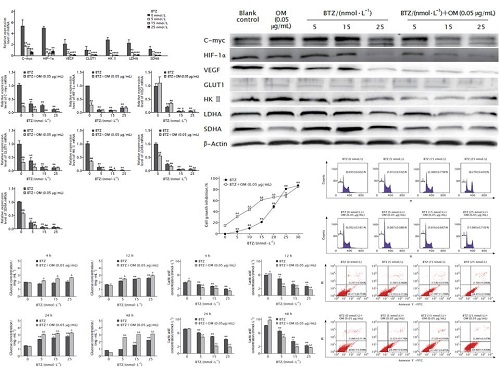
The result of CCK-8 method showed that treatment with different concentrations of BTZ could inhibit the proliferation of Raji cells in a dose-dependent manner (all P < 0.01), the inhibition effect was significantly enhanced at lower initial concentrations of BTZ (5, 10, 15 and 20 nmol/L) in combination with OM (all P < 0.01). The expression levels of C-myc, HIF-1α, VEGF, GLUT1, HKII, LDHA and SDHA mRNAs and proteins were suppressed by BTZ, and the expression levels were further down-regulated due to treatment with BTZ in combination with OM (all P < 0.05). The inhibition of glucose consumption and lactic acid generation induced by BTZ in combination with OM was significantly enhanced as compared with those induced by BTZ alone (both P < 0.05). The result of FCM showed that BTZ could induce apoptosis of Raji cells in a dose-dependent manner, and the cell cycle was arrested in G2/M phase under a relatively high concentration of BTZ (25 nmol/L). Furthermore, when BTZ was used in combination with aerobic oxidation inhibitor OM, significant synergistic effect was observed; besides, the cell cycle was arrested at G0/G1phase instead.
Conclusion
BTZ can hinder the glycolysis pathway in Burkitt lymphoma cell line Raji, which can also be enhanced by the involvement of aerobic oxidation inhibitor OM, acting as a synergistic inhibition. The mechanism of this synergy may be interpreted as inhibiting the dual metabolic pathway, namely glycolysis and aerobic oxidation. This inhibition of dual metabolic pathway may imply a novel approach to the treatment of Burkitt lymphoma.
Session topic: E-poster
Keyword(s): Bortezomib, Burkitt's lymphoma, NHL, Targeted therapy
Type: Eposter Presentation
Background
Caner and non-malignant cells vary vitally with respect to their metabolic pathway. Consuming elevated levels of glucose from a frequently nutrient-poor environment in order to satiate anabolic respiratory reactions, is a sufficiently prevalent phenomenon of cancer cell metabolic adaptions, which has been conceptualized as the “Warburg effect”. However, the molecular mechanism underlying this metabolic reprogramming is obscure. Recent investigations propose that the oncogene-directed metabolic reprogramming, rather than the permanent malfunction of mitochondrial oxidative phosphorylation (OXPHOS), may have profound effects on aerobic glycolysis. The biochemical aspects of the Warburg effect outline a strong explanation for the cause of cancer cell proliferation. However, the more reliant of malignant cells manifest on glycolysis, the more vulnerable to drugs targeting this pathway they will be. Prognosis of Burkitt lymphoma, the most aggressive B-cell lymphoma, is poor even in the Rituximab era. Therefore, targeting the aberrant metabolic pathway rather than illimitably increase the dose of chemotherapy prompts a highly desirable strategy for treating this aggressive lymphoma. Bortezomib is the first proteasome inhibitor approved for treating multiple myeloma and mantel cell lymphoma. In addition to the well-known mechanism on proteasome inhibition, bortezomib is supposed to have effects on the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway, which is deemed crucial to underpin the glycolysis of malignant cells. Given the central role of glycolysis in cancer metabolism, we sought to explore the effect of bortezomib on tumor cell metabolism in Burkitt lymphoma. Considering the fact that even counteracting glycolysis, cancer cells shows low-glucose resistance, which may associate with an increased compensatory upregulation of mitochondrial OXPHOS, the present study was undertaken to investigate the potential of using Bortezomib in combination with OXPHOS inhibitor as a novel therapy for Burkitt lymphoma.
Aims
To investigate the effect of bortezomib (BTZ) in combination with aerobic oxidation inhibitor oligomycin (OM) on proliferation and apoptosis of Burkitt lymphoma cell line Raji, and to explore its possible molecular mechanism.
Methods
Raji cells were treated with different concentrations of BTZ (0, 5, 10, 15, 20, 25 and 30 nmol/L) alone or in combination with OM (0.05 µg/mL). Cell proliferation was detected by CCK-8 method. The mRNA and protein expression levels of oncogene C-myc, hypoxia inducible factor-1α (HIF-1α) and its target genes vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT1), as well as key enzymes and proteins related to glycolysis pathway including hexokinase II (HKII), lactic dehydrogenase (LDHA) and succinate dehydrogenase (SDHA) were detected by real-time fluorescent quantitative PCR and Western blotting, respectively. Glucose consumption and lactic acid generation were examined by Glucose (hexokinase, HK) Assay Kit and Lactate Assay Kit, respectively. Apoptosis and cell cycle distribution were analyzed by FCM.
Results
The result of CCK-8 method showed that treatment with different concentrations of BTZ could inhibit the proliferation of Raji cells in a dose-dependent manner (all P < 0.01), the inhibition effect was significantly enhanced at lower initial concentrations of BTZ (5, 10, 15 and 20 nmol/L) in combination with OM (all P < 0.01). The expression levels of C-myc, HIF-1α, VEGF, GLUT1, HKII, LDHA and SDHA mRNAs and proteins were suppressed by BTZ, and the expression levels were further down-regulated due to treatment with BTZ in combination with OM (all P < 0.05). The inhibition of glucose consumption and lactic acid generation induced by BTZ in combination with OM was significantly enhanced as compared with those induced by BTZ alone (both P < 0.05). The result of FCM showed that BTZ could induce apoptosis of Raji cells in a dose-dependent manner, and the cell cycle was arrested in G2/M phase under a relatively high concentration of BTZ (25 nmol/L). Furthermore, when BTZ was used in combination with aerobic oxidation inhibitor OM, significant synergistic effect was observed; besides, the cell cycle was arrested at G0/G1phase instead.
Conclusion
BTZ can hinder the glycolysis pathway in Burkitt lymphoma cell line Raji, which can also be enhanced by the involvement of aerobic oxidation inhibitor OM, acting as a synergistic inhibition. The mechanism of this synergy may be interpreted as inhibiting the dual metabolic pathway, namely glycolysis and aerobic oxidation. This inhibition of dual metabolic pathway may imply a novel approach to the treatment of Burkitt lymphoma.

Session topic: E-poster
Keyword(s): Bortezomib, Burkitt's lymphoma, NHL, Targeted therapy
Abstract: E1398
Type: Eposter Presentation
Background
Caner and non-malignant cells vary vitally with respect to their metabolic pathway. Consuming elevated levels of glucose from a frequently nutrient-poor environment in order to satiate anabolic respiratory reactions, is a sufficiently prevalent phenomenon of cancer cell metabolic adaptions, which has been conceptualized as the “Warburg effect”. However, the molecular mechanism underlying this metabolic reprogramming is obscure. Recent investigations propose that the oncogene-directed metabolic reprogramming, rather than the permanent malfunction of mitochondrial oxidative phosphorylation (OXPHOS), may have profound effects on aerobic glycolysis. The biochemical aspects of the Warburg effect outline a strong explanation for the cause of cancer cell proliferation. However, the more reliant of malignant cells manifest on glycolysis, the more vulnerable to drugs targeting this pathway they will be. Prognosis of Burkitt lymphoma, the most aggressive B-cell lymphoma, is poor even in the Rituximab era. Therefore, targeting the aberrant metabolic pathway rather than illimitably increase the dose of chemotherapy prompts a highly desirable strategy for treating this aggressive lymphoma. Bortezomib is the first proteasome inhibitor approved for treating multiple myeloma and mantel cell lymphoma. In addition to the well-known mechanism on proteasome inhibition, bortezomib is supposed to have effects on the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway, which is deemed crucial to underpin the glycolysis of malignant cells. Given the central role of glycolysis in cancer metabolism, we sought to explore the effect of bortezomib on tumor cell metabolism in Burkitt lymphoma. Considering the fact that even counteracting glycolysis, cancer cells shows low-glucose resistance, which may associate with an increased compensatory upregulation of mitochondrial OXPHOS, the present study was undertaken to investigate the potential of using Bortezomib in combination with OXPHOS inhibitor as a novel therapy for Burkitt lymphoma.
Aims
To investigate the effect of bortezomib (BTZ) in combination with aerobic oxidation inhibitor oligomycin (OM) on proliferation and apoptosis of Burkitt lymphoma cell line Raji, and to explore its possible molecular mechanism.
Methods
Raji cells were treated with different concentrations of BTZ (0, 5, 10, 15, 20, 25 and 30 nmol/L) alone or in combination with OM (0.05 µg/mL). Cell proliferation was detected by CCK-8 method. The mRNA and protein expression levels of oncogene C-myc, hypoxia inducible factor-1α (HIF-1α) and its target genes vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT1), as well as key enzymes and proteins related to glycolysis pathway including hexokinase II (HKII), lactic dehydrogenase (LDHA) and succinate dehydrogenase (SDHA) were detected by real-time fluorescent quantitative PCR and Western blotting, respectively. Glucose consumption and lactic acid generation were examined by Glucose (hexokinase, HK) Assay Kit and Lactate Assay Kit, respectively. Apoptosis and cell cycle distribution were analyzed by FCM.
Results
The result of CCK-8 method showed that treatment with different concentrations of BTZ could inhibit the proliferation of Raji cells in a dose-dependent manner (all P < 0.01), the inhibition effect was significantly enhanced at lower initial concentrations of BTZ (5, 10, 15 and 20 nmol/L) in combination with OM (all P < 0.01). The expression levels of C-myc, HIF-1α, VEGF, GLUT1, HKII, LDHA and SDHA mRNAs and proteins were suppressed by BTZ, and the expression levels were further down-regulated due to treatment with BTZ in combination with OM (all P < 0.05). The inhibition of glucose consumption and lactic acid generation induced by BTZ in combination with OM was significantly enhanced as compared with those induced by BTZ alone (both P < 0.05). The result of FCM showed that BTZ could induce apoptosis of Raji cells in a dose-dependent manner, and the cell cycle was arrested in G2/M phase under a relatively high concentration of BTZ (25 nmol/L). Furthermore, when BTZ was used in combination with aerobic oxidation inhibitor OM, significant synergistic effect was observed; besides, the cell cycle was arrested at G0/G1phase instead.
Conclusion
BTZ can hinder the glycolysis pathway in Burkitt lymphoma cell line Raji, which can also be enhanced by the involvement of aerobic oxidation inhibitor OM, acting as a synergistic inhibition. The mechanism of this synergy may be interpreted as inhibiting the dual metabolic pathway, namely glycolysis and aerobic oxidation. This inhibition of dual metabolic pathway may imply a novel approach to the treatment of Burkitt lymphoma.

Session topic: E-poster
Keyword(s): Bortezomib, Burkitt's lymphoma, NHL, Targeted therapy
Type: Eposter Presentation
Background
Caner and non-malignant cells vary vitally with respect to their metabolic pathway. Consuming elevated levels of glucose from a frequently nutrient-poor environment in order to satiate anabolic respiratory reactions, is a sufficiently prevalent phenomenon of cancer cell metabolic adaptions, which has been conceptualized as the “Warburg effect”. However, the molecular mechanism underlying this metabolic reprogramming is obscure. Recent investigations propose that the oncogene-directed metabolic reprogramming, rather than the permanent malfunction of mitochondrial oxidative phosphorylation (OXPHOS), may have profound effects on aerobic glycolysis. The biochemical aspects of the Warburg effect outline a strong explanation for the cause of cancer cell proliferation. However, the more reliant of malignant cells manifest on glycolysis, the more vulnerable to drugs targeting this pathway they will be. Prognosis of Burkitt lymphoma, the most aggressive B-cell lymphoma, is poor even in the Rituximab era. Therefore, targeting the aberrant metabolic pathway rather than illimitably increase the dose of chemotherapy prompts a highly desirable strategy for treating this aggressive lymphoma. Bortezomib is the first proteasome inhibitor approved for treating multiple myeloma and mantel cell lymphoma. In addition to the well-known mechanism on proteasome inhibition, bortezomib is supposed to have effects on the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway, which is deemed crucial to underpin the glycolysis of malignant cells. Given the central role of glycolysis in cancer metabolism, we sought to explore the effect of bortezomib on tumor cell metabolism in Burkitt lymphoma. Considering the fact that even counteracting glycolysis, cancer cells shows low-glucose resistance, which may associate with an increased compensatory upregulation of mitochondrial OXPHOS, the present study was undertaken to investigate the potential of using Bortezomib in combination with OXPHOS inhibitor as a novel therapy for Burkitt lymphoma.
Aims
To investigate the effect of bortezomib (BTZ) in combination with aerobic oxidation inhibitor oligomycin (OM) on proliferation and apoptosis of Burkitt lymphoma cell line Raji, and to explore its possible molecular mechanism.
Methods
Raji cells were treated with different concentrations of BTZ (0, 5, 10, 15, 20, 25 and 30 nmol/L) alone or in combination with OM (0.05 µg/mL). Cell proliferation was detected by CCK-8 method. The mRNA and protein expression levels of oncogene C-myc, hypoxia inducible factor-1α (HIF-1α) and its target genes vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT1), as well as key enzymes and proteins related to glycolysis pathway including hexokinase II (HKII), lactic dehydrogenase (LDHA) and succinate dehydrogenase (SDHA) were detected by real-time fluorescent quantitative PCR and Western blotting, respectively. Glucose consumption and lactic acid generation were examined by Glucose (hexokinase, HK) Assay Kit and Lactate Assay Kit, respectively. Apoptosis and cell cycle distribution were analyzed by FCM.
Results
The result of CCK-8 method showed that treatment with different concentrations of BTZ could inhibit the proliferation of Raji cells in a dose-dependent manner (all P < 0.01), the inhibition effect was significantly enhanced at lower initial concentrations of BTZ (5, 10, 15 and 20 nmol/L) in combination with OM (all P < 0.01). The expression levels of C-myc, HIF-1α, VEGF, GLUT1, HKII, LDHA and SDHA mRNAs and proteins were suppressed by BTZ, and the expression levels were further down-regulated due to treatment with BTZ in combination with OM (all P < 0.05). The inhibition of glucose consumption and lactic acid generation induced by BTZ in combination with OM was significantly enhanced as compared with those induced by BTZ alone (both P < 0.05). The result of FCM showed that BTZ could induce apoptosis of Raji cells in a dose-dependent manner, and the cell cycle was arrested in G2/M phase under a relatively high concentration of BTZ (25 nmol/L). Furthermore, when BTZ was used in combination with aerobic oxidation inhibitor OM, significant synergistic effect was observed; besides, the cell cycle was arrested at G0/G1phase instead.
Conclusion
BTZ can hinder the glycolysis pathway in Burkitt lymphoma cell line Raji, which can also be enhanced by the involvement of aerobic oxidation inhibitor OM, acting as a synergistic inhibition. The mechanism of this synergy may be interpreted as inhibiting the dual metabolic pathway, namely glycolysis and aerobic oxidation. This inhibition of dual metabolic pathway may imply a novel approach to the treatment of Burkitt lymphoma.

Session topic: E-poster
Keyword(s): Bortezomib, Burkitt's lymphoma, NHL, Targeted therapy
{{ help_message }}
{{filter}}


