INTERRUPTION OF CCL20-CCR6 INTERACTION INHIBITS METASTASIS OF ADVANCED CUTANEOUS T-CELL LYMPHOMA
(Abstract release date: 05/19/16)
EHA Library. Tagawa H. 06/09/16; 132928; E1379

Dr. Hiroyuki Tagawa
Contributions
Contributions
Abstract
Abstract: E1379
Type: Eposter Presentation
Background
The expression of interleukin-22 (IL-22), chemokine receptor CCR6, and its ligand CCL20 is upregulated in advanced Cutaneous T-cell lymphoma (CTCL) (Miyagaki et al., Clin Cancer Res 2011). We have also shown that a non-coding RNA, microRNA-150 (miR-150), is silenced in advanced CTCL, and that the miR-150 downregulates CCR6 directly and CCL20 indirectly. Based on these data, we hypothesized that continuous CCR6 and CCL20 upregulation might lead to continuous CCL20-CCR6 interaction in CTCL cells and in turn, lead to metastasis to distal organs in a nutrition-dependent manner. We further found that IL22RA1, one of the IL-22 receptor subunits, was aberrantly overexpressed in CTCL, and that its knockdown decreased CCL20 production. These results suggested that the IL-22 produced by the CTCL cells might activate the IL-22 receptor in these cells, leading to the activation of downstream targets and subsequently increasing the transcription of CCL20 (Ito et al., Blood 2014). However, we could not determine the downstream cascade of IL-22 that mediates CCL20 transcription activation.
Aims
Aim of this study is to determine whether 1) CCR6-CCL20 interaction is actually functional in advanced CTCL cells representing metastatic capability, and 2) which transcriptional factor(s) might activate CCL20 activation.
Methods
Immunohistochemistry of p-STAT3 and CCR6 were performed for five patients with Mycosis Fungoides patients of early and advanced phase of the same individual. CTCL cell lines such as My-La, HH MJ and HUT78 were used for in vitro experiment. Transient knockdown of IL22RA1, CCR6, and CCL20 was conducted for detecting functional analysis of CTCL cells. To determine whether the transient knockdown of STAT3, CCL20, or CCR6 or treatment with neutralizing CCL20 antibody could reduce the migration ability of CTCL cells, we conducted an in vitro migration assay. To examine the in vivo effect of neutralizing CCL20 antibody, we used NOD/Shi-scid IL-2γnul mice inoculated with CTCL cells (namely CTCL mice). Written informed consent was obtained from all patients prior to collection of specimens, in keeping with all institutional policies and according to the Declaration of Helsinki. Samples were collected under a protocol approved by the Institutional Review Boards of Akita University and University of Tokyo.
Results
We demonstrated increased STAT3 expression during the progression of primary CTCL. STAT3 was spontaneously activated and mediated the transcription of CCL20 in CTCL cell lines. However STAT3 was not spontaneously activated in mantle cell lymphoma cell lines because these cell lines required upstream stimulation of IL-22 for activation of CCL20. In vitro migration assay demonstrated that all treatments reduced the nutrition-dependent migration activity of CTCL cells. Notably, treatment with neutralizing CCL20 antibody reduced the migration ability of the cells without decreasing the expression of CCL20 and CCR6. This demonstrates that the CCL20-CCR6 interaction is actually functional in metastatic CTCL cells. In vivo administration of neutralizing CCL20 antibody significantly prolonged the survival of the CTCL mice.
Conclusion
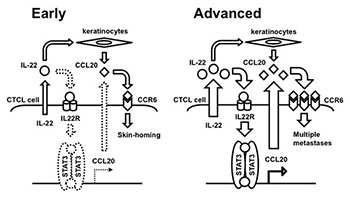
These findings suggested that automatic activation of the STAT3/CCL20/CCR6 cascade was actually involved in advanced CTCL lymphomagenesis. On the other hand, activation of STAT3 might require upstream stimulation of IL-22 in early CTCL (see image Figure). Thus, disruption of CCL20-CCR6 interaction could be a key therapeutic strategy against advanced CTCL.
Session topic: E-poster
Keyword(s): Chemokine, Chemokine receptor, Cutaneous T-cell lymphoma, Lymphoma therapy
Type: Eposter Presentation
Background
The expression of interleukin-22 (IL-22), chemokine receptor CCR6, and its ligand CCL20 is upregulated in advanced Cutaneous T-cell lymphoma (CTCL) (Miyagaki et al., Clin Cancer Res 2011). We have also shown that a non-coding RNA, microRNA-150 (miR-150), is silenced in advanced CTCL, and that the miR-150 downregulates CCR6 directly and CCL20 indirectly. Based on these data, we hypothesized that continuous CCR6 and CCL20 upregulation might lead to continuous CCL20-CCR6 interaction in CTCL cells and in turn, lead to metastasis to distal organs in a nutrition-dependent manner. We further found that IL22RA1, one of the IL-22 receptor subunits, was aberrantly overexpressed in CTCL, and that its knockdown decreased CCL20 production. These results suggested that the IL-22 produced by the CTCL cells might activate the IL-22 receptor in these cells, leading to the activation of downstream targets and subsequently increasing the transcription of CCL20 (Ito et al., Blood 2014). However, we could not determine the downstream cascade of IL-22 that mediates CCL20 transcription activation.
Aims
Aim of this study is to determine whether 1) CCR6-CCL20 interaction is actually functional in advanced CTCL cells representing metastatic capability, and 2) which transcriptional factor(s) might activate CCL20 activation.
Methods
Immunohistochemistry of p-STAT3 and CCR6 were performed for five patients with Mycosis Fungoides patients of early and advanced phase of the same individual. CTCL cell lines such as My-La, HH MJ and HUT78 were used for in vitro experiment. Transient knockdown of IL22RA1, CCR6, and CCL20 was conducted for detecting functional analysis of CTCL cells. To determine whether the transient knockdown of STAT3, CCL20, or CCR6 or treatment with neutralizing CCL20 antibody could reduce the migration ability of CTCL cells, we conducted an in vitro migration assay. To examine the in vivo effect of neutralizing CCL20 antibody, we used NOD/Shi-scid IL-2γnul mice inoculated with CTCL cells (namely CTCL mice). Written informed consent was obtained from all patients prior to collection of specimens, in keeping with all institutional policies and according to the Declaration of Helsinki. Samples were collected under a protocol approved by the Institutional Review Boards of Akita University and University of Tokyo.
Results
We demonstrated increased STAT3 expression during the progression of primary CTCL. STAT3 was spontaneously activated and mediated the transcription of CCL20 in CTCL cell lines. However STAT3 was not spontaneously activated in mantle cell lymphoma cell lines because these cell lines required upstream stimulation of IL-22 for activation of CCL20. In vitro migration assay demonstrated that all treatments reduced the nutrition-dependent migration activity of CTCL cells. Notably, treatment with neutralizing CCL20 antibody reduced the migration ability of the cells without decreasing the expression of CCL20 and CCR6. This demonstrates that the CCL20-CCR6 interaction is actually functional in metastatic CTCL cells. In vivo administration of neutralizing CCL20 antibody significantly prolonged the survival of the CTCL mice.
Conclusion
These findings suggested that automatic activation of the STAT3/CCL20/CCR6 cascade was actually involved in advanced CTCL lymphomagenesis. On the other hand, activation of STAT3 might require upstream stimulation of IL-22 in early CTCL (see image Figure). Thus, disruption of CCL20-CCR6 interaction could be a key therapeutic strategy against advanced CTCL.

Session topic: E-poster
Keyword(s): Chemokine, Chemokine receptor, Cutaneous T-cell lymphoma, Lymphoma therapy
Abstract: E1379
Type: Eposter Presentation
Background
The expression of interleukin-22 (IL-22), chemokine receptor CCR6, and its ligand CCL20 is upregulated in advanced Cutaneous T-cell lymphoma (CTCL) (Miyagaki et al., Clin Cancer Res 2011). We have also shown that a non-coding RNA, microRNA-150 (miR-150), is silenced in advanced CTCL, and that the miR-150 downregulates CCR6 directly and CCL20 indirectly. Based on these data, we hypothesized that continuous CCR6 and CCL20 upregulation might lead to continuous CCL20-CCR6 interaction in CTCL cells and in turn, lead to metastasis to distal organs in a nutrition-dependent manner. We further found that IL22RA1, one of the IL-22 receptor subunits, was aberrantly overexpressed in CTCL, and that its knockdown decreased CCL20 production. These results suggested that the IL-22 produced by the CTCL cells might activate the IL-22 receptor in these cells, leading to the activation of downstream targets and subsequently increasing the transcription of CCL20 (Ito et al., Blood 2014). However, we could not determine the downstream cascade of IL-22 that mediates CCL20 transcription activation.
Aims
Aim of this study is to determine whether 1) CCR6-CCL20 interaction is actually functional in advanced CTCL cells representing metastatic capability, and 2) which transcriptional factor(s) might activate CCL20 activation.
Methods
Immunohistochemistry of p-STAT3 and CCR6 were performed for five patients with Mycosis Fungoides patients of early and advanced phase of the same individual. CTCL cell lines such as My-La, HH MJ and HUT78 were used for in vitro experiment. Transient knockdown of IL22RA1, CCR6, and CCL20 was conducted for detecting functional analysis of CTCL cells. To determine whether the transient knockdown of STAT3, CCL20, or CCR6 or treatment with neutralizing CCL20 antibody could reduce the migration ability of CTCL cells, we conducted an in vitro migration assay. To examine the in vivo effect of neutralizing CCL20 antibody, we used NOD/Shi-scid IL-2γnul mice inoculated with CTCL cells (namely CTCL mice). Written informed consent was obtained from all patients prior to collection of specimens, in keeping with all institutional policies and according to the Declaration of Helsinki. Samples were collected under a protocol approved by the Institutional Review Boards of Akita University and University of Tokyo.
Results
We demonstrated increased STAT3 expression during the progression of primary CTCL. STAT3 was spontaneously activated and mediated the transcription of CCL20 in CTCL cell lines. However STAT3 was not spontaneously activated in mantle cell lymphoma cell lines because these cell lines required upstream stimulation of IL-22 for activation of CCL20. In vitro migration assay demonstrated that all treatments reduced the nutrition-dependent migration activity of CTCL cells. Notably, treatment with neutralizing CCL20 antibody reduced the migration ability of the cells without decreasing the expression of CCL20 and CCR6. This demonstrates that the CCL20-CCR6 interaction is actually functional in metastatic CTCL cells. In vivo administration of neutralizing CCL20 antibody significantly prolonged the survival of the CTCL mice.
Conclusion
These findings suggested that automatic activation of the STAT3/CCL20/CCR6 cascade was actually involved in advanced CTCL lymphomagenesis. On the other hand, activation of STAT3 might require upstream stimulation of IL-22 in early CTCL (see image Figure). Thus, disruption of CCL20-CCR6 interaction could be a key therapeutic strategy against advanced CTCL.

Session topic: E-poster
Keyword(s): Chemokine, Chemokine receptor, Cutaneous T-cell lymphoma, Lymphoma therapy
Type: Eposter Presentation
Background
The expression of interleukin-22 (IL-22), chemokine receptor CCR6, and its ligand CCL20 is upregulated in advanced Cutaneous T-cell lymphoma (CTCL) (Miyagaki et al., Clin Cancer Res 2011). We have also shown that a non-coding RNA, microRNA-150 (miR-150), is silenced in advanced CTCL, and that the miR-150 downregulates CCR6 directly and CCL20 indirectly. Based on these data, we hypothesized that continuous CCR6 and CCL20 upregulation might lead to continuous CCL20-CCR6 interaction in CTCL cells and in turn, lead to metastasis to distal organs in a nutrition-dependent manner. We further found that IL22RA1, one of the IL-22 receptor subunits, was aberrantly overexpressed in CTCL, and that its knockdown decreased CCL20 production. These results suggested that the IL-22 produced by the CTCL cells might activate the IL-22 receptor in these cells, leading to the activation of downstream targets and subsequently increasing the transcription of CCL20 (Ito et al., Blood 2014). However, we could not determine the downstream cascade of IL-22 that mediates CCL20 transcription activation.
Aims
Aim of this study is to determine whether 1) CCR6-CCL20 interaction is actually functional in advanced CTCL cells representing metastatic capability, and 2) which transcriptional factor(s) might activate CCL20 activation.
Methods
Immunohistochemistry of p-STAT3 and CCR6 were performed for five patients with Mycosis Fungoides patients of early and advanced phase of the same individual. CTCL cell lines such as My-La, HH MJ and HUT78 were used for in vitro experiment. Transient knockdown of IL22RA1, CCR6, and CCL20 was conducted for detecting functional analysis of CTCL cells. To determine whether the transient knockdown of STAT3, CCL20, or CCR6 or treatment with neutralizing CCL20 antibody could reduce the migration ability of CTCL cells, we conducted an in vitro migration assay. To examine the in vivo effect of neutralizing CCL20 antibody, we used NOD/Shi-scid IL-2γnul mice inoculated with CTCL cells (namely CTCL mice). Written informed consent was obtained from all patients prior to collection of specimens, in keeping with all institutional policies and according to the Declaration of Helsinki. Samples were collected under a protocol approved by the Institutional Review Boards of Akita University and University of Tokyo.
Results
We demonstrated increased STAT3 expression during the progression of primary CTCL. STAT3 was spontaneously activated and mediated the transcription of CCL20 in CTCL cell lines. However STAT3 was not spontaneously activated in mantle cell lymphoma cell lines because these cell lines required upstream stimulation of IL-22 for activation of CCL20. In vitro migration assay demonstrated that all treatments reduced the nutrition-dependent migration activity of CTCL cells. Notably, treatment with neutralizing CCL20 antibody reduced the migration ability of the cells without decreasing the expression of CCL20 and CCR6. This demonstrates that the CCL20-CCR6 interaction is actually functional in metastatic CTCL cells. In vivo administration of neutralizing CCL20 antibody significantly prolonged the survival of the CTCL mice.
Conclusion
These findings suggested that automatic activation of the STAT3/CCL20/CCR6 cascade was actually involved in advanced CTCL lymphomagenesis. On the other hand, activation of STAT3 might require upstream stimulation of IL-22 in early CTCL (see image Figure). Thus, disruption of CCL20-CCR6 interaction could be a key therapeutic strategy against advanced CTCL.

Session topic: E-poster
Keyword(s): Chemokine, Chemokine receptor, Cutaneous T-cell lymphoma, Lymphoma therapy
{{ help_message }}
{{filter}}


