RUXOLITINIB TREATMENT FOLLOWING INTERFERON IN PATIENTS WITH POLYCYTHEMIA VERA: AN ANALYSIS FROM THE RESPONSE TRIAL
(Abstract release date: 05/19/16)
EHA Library. Kiladjian J. 06/09/16; 132909; E1360

Prof. Jean-Jacques Kiladjian
Contributions
Contributions
Abstract
Abstract: E1360
Type: Eposter Presentation
Background
Polycythemia vera (PV) is characterized by excessive proliferation of erythroid, myeloid, and megakaryocytic components in the bone resulting in an increased risk of thromboembolic events, cardiovascular complications, and disease progression. Both hydroxyurea (HU) and interferon-alpha (IFN) are used as first-line treatment for high-risk pts with PV but pts may become resistant/intolerant (Barbui. JCO 2011; 29(6):761-770). The recent phase 3 RESPONSE study showed that ruxolitinib (RUX) was superior to best available therapy (BAT) following HU resistance/intolerance; however, little information has been presented on the sub-group of pts treated with IFN in BAT arm.
Aims
This subgroup analysis of RESPONSE study evaluates the safety and efficacy of pts treated with IFN in the randomized BAT arm before and after crossover to RUX.
Methods
RESPONSE, an open-label, phase 3 study enrolled pts with PV, who were resistant to or intolerant of HU per modified European LeukemiaNet criteria, had splenomegaly and required phlebotomy (PBT) to control hematocrit (HCT). Pts were randomized 1:1 to RUX (110 pts) 10 mg bid or BAT (112 pts), of which 13 pts received at least one dose of IFN during the randomized treatment phase. Pts were evaluated for HCT control (<45%) without PBT; spleen response (≥35% reduction in spleen volume from baseline by magnetic resonance imaging); and complete hematologic response [CHR: HCT<45%, white blood cell (WBC) ≤10 × 109/L, platelet (PLT) count ≤400 x 109/L]. JAK2V617F allele burden, adverse events (AEs) and hematologic abnormalities were assessed for randomized and crossover treatment.
Results
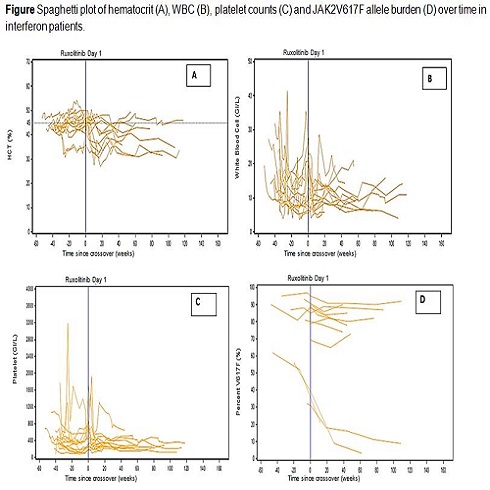
Baseline demographics were comparable among RUX (N=110) and IFN-treated pts of the BAT arm (N=13). Three pts (23%) received IFN at some time prior to inclusion in the RESPONSE trial. On trial, 9 pts were treated with PEG-IFN and 4 pts were treated with non-PEG-IFN at varying doses. All IFN pts discontinued and crossed over to RUX due to lack of efficacy (11 pts crossed over prior to wk 48 and 2 pts after wk 48). No pt treated with IFN achieved spleen response and only 23% of pts achieved HCT control without PBT from wk 8 to 32 (40% and 60% while on RUX therapy, respectively). Thirty-eight percent, 31%, and 31% of pts required no PBT, 1 PBT, and ≥2 PBT, respectively, from wk 8 to 32 of randomized treatment and only 15% of pts achieved CHR at wk 32. Following crossover to RUX, 62% of pts achieved spleen response and PBT requirements decreased (64% of pts did not require any PBT and no pt required ≥2 PBT from wk 32 to 80 after switching to RUX). A majority of pts randomized to IFN had modest reductions in WBC, PLTs, and JAK2V617F allele burden over the course of treatment (figure), with most pts showing further reduction after crossover to RUX. The rates and incidence of hematologic abnormalities (WBC, PLT, and neutrophils) reduced after crossover to RUX with the exception of hemoglobin (prior to crossover: grade 1 [N=2] and grade 2 [N=1]; after crossover: grade 1 [N=5] and grade 2 [N=2]). Similarly, rates and incidence of nonhematologic AEs (systemic, metabolic, gout, pruritus, and fatigue) reduced after crossover to RUX with the exception of infections (primarily grade 1 or 2). Four pts discontinued crossover treatment due to AE (N=3) or pt decision (N=1). One death due to CNS hemorrhage occurred after crossover to RUX (not related to study drug).
Conclusion
In the HU-resistant/intolerant PV population of the RESPONSE study, no pt treated with IFN achieved spleen response and a limited number of pts achieved HCT control. However, following crossover to RUX, pts showed improvement in hematologic and spleen response with an overall reduction in PBT procedures and a majority of pts being able to achieve spleen response.
Session topic: E-poster
Keyword(s): Interferon, Myeloproliferative disorder, Polycythemia vera, Ruxolitinib
Type: Eposter Presentation
Background
Polycythemia vera (PV) is characterized by excessive proliferation of erythroid, myeloid, and megakaryocytic components in the bone resulting in an increased risk of thromboembolic events, cardiovascular complications, and disease progression. Both hydroxyurea (HU) and interferon-alpha (IFN) are used as first-line treatment for high-risk pts with PV but pts may become resistant/intolerant (Barbui. JCO 2011; 29(6):761-770). The recent phase 3 RESPONSE study showed that ruxolitinib (RUX) was superior to best available therapy (BAT) following HU resistance/intolerance; however, little information has been presented on the sub-group of pts treated with IFN in BAT arm.
Aims
This subgroup analysis of RESPONSE study evaluates the safety and efficacy of pts treated with IFN in the randomized BAT arm before and after crossover to RUX.
Methods
RESPONSE, an open-label, phase 3 study enrolled pts with PV, who were resistant to or intolerant of HU per modified European LeukemiaNet criteria, had splenomegaly and required phlebotomy (PBT) to control hematocrit (HCT). Pts were randomized 1:1 to RUX (110 pts) 10 mg bid or BAT (112 pts), of which 13 pts received at least one dose of IFN during the randomized treatment phase. Pts were evaluated for HCT control (<45%) without PBT; spleen response (≥35% reduction in spleen volume from baseline by magnetic resonance imaging); and complete hematologic response [CHR: HCT<45%, white blood cell (WBC) ≤10 × 109/L, platelet (PLT) count ≤400 x 109/L]. JAK2V617F allele burden, adverse events (AEs) and hematologic abnormalities were assessed for randomized and crossover treatment.
Results
Baseline demographics were comparable among RUX (N=110) and IFN-treated pts of the BAT arm (N=13). Three pts (23%) received IFN at some time prior to inclusion in the RESPONSE trial. On trial, 9 pts were treated with PEG-IFN and 4 pts were treated with non-PEG-IFN at varying doses. All IFN pts discontinued and crossed over to RUX due to lack of efficacy (11 pts crossed over prior to wk 48 and 2 pts after wk 48). No pt treated with IFN achieved spleen response and only 23% of pts achieved HCT control without PBT from wk 8 to 32 (40% and 60% while on RUX therapy, respectively). Thirty-eight percent, 31%, and 31% of pts required no PBT, 1 PBT, and ≥2 PBT, respectively, from wk 8 to 32 of randomized treatment and only 15% of pts achieved CHR at wk 32. Following crossover to RUX, 62% of pts achieved spleen response and PBT requirements decreased (64% of pts did not require any PBT and no pt required ≥2 PBT from wk 32 to 80 after switching to RUX). A majority of pts randomized to IFN had modest reductions in WBC, PLTs, and JAK2V617F allele burden over the course of treatment (figure), with most pts showing further reduction after crossover to RUX. The rates and incidence of hematologic abnormalities (WBC, PLT, and neutrophils) reduced after crossover to RUX with the exception of hemoglobin (prior to crossover: grade 1 [N=2] and grade 2 [N=1]; after crossover: grade 1 [N=5] and grade 2 [N=2]). Similarly, rates and incidence of nonhematologic AEs (systemic, metabolic, gout, pruritus, and fatigue) reduced after crossover to RUX with the exception of infections (primarily grade 1 or 2). Four pts discontinued crossover treatment due to AE (N=3) or pt decision (N=1). One death due to CNS hemorrhage occurred after crossover to RUX (not related to study drug).
Conclusion
In the HU-resistant/intolerant PV population of the RESPONSE study, no pt treated with IFN achieved spleen response and a limited number of pts achieved HCT control. However, following crossover to RUX, pts showed improvement in hematologic and spleen response with an overall reduction in PBT procedures and a majority of pts being able to achieve spleen response.

Session topic: E-poster
Keyword(s): Interferon, Myeloproliferative disorder, Polycythemia vera, Ruxolitinib
Abstract: E1360
Type: Eposter Presentation
Background
Polycythemia vera (PV) is characterized by excessive proliferation of erythroid, myeloid, and megakaryocytic components in the bone resulting in an increased risk of thromboembolic events, cardiovascular complications, and disease progression. Both hydroxyurea (HU) and interferon-alpha (IFN) are used as first-line treatment for high-risk pts with PV but pts may become resistant/intolerant (Barbui. JCO 2011; 29(6):761-770). The recent phase 3 RESPONSE study showed that ruxolitinib (RUX) was superior to best available therapy (BAT) following HU resistance/intolerance; however, little information has been presented on the sub-group of pts treated with IFN in BAT arm.
Aims
This subgroup analysis of RESPONSE study evaluates the safety and efficacy of pts treated with IFN in the randomized BAT arm before and after crossover to RUX.
Methods
RESPONSE, an open-label, phase 3 study enrolled pts with PV, who were resistant to or intolerant of HU per modified European LeukemiaNet criteria, had splenomegaly and required phlebotomy (PBT) to control hematocrit (HCT). Pts were randomized 1:1 to RUX (110 pts) 10 mg bid or BAT (112 pts), of which 13 pts received at least one dose of IFN during the randomized treatment phase. Pts were evaluated for HCT control (<45%) without PBT; spleen response (≥35% reduction in spleen volume from baseline by magnetic resonance imaging); and complete hematologic response [CHR: HCT<45%, white blood cell (WBC) ≤10 × 109/L, platelet (PLT) count ≤400 x 109/L]. JAK2V617F allele burden, adverse events (AEs) and hematologic abnormalities were assessed for randomized and crossover treatment.
Results
Baseline demographics were comparable among RUX (N=110) and IFN-treated pts of the BAT arm (N=13). Three pts (23%) received IFN at some time prior to inclusion in the RESPONSE trial. On trial, 9 pts were treated with PEG-IFN and 4 pts were treated with non-PEG-IFN at varying doses. All IFN pts discontinued and crossed over to RUX due to lack of efficacy (11 pts crossed over prior to wk 48 and 2 pts after wk 48). No pt treated with IFN achieved spleen response and only 23% of pts achieved HCT control without PBT from wk 8 to 32 (40% and 60% while on RUX therapy, respectively). Thirty-eight percent, 31%, and 31% of pts required no PBT, 1 PBT, and ≥2 PBT, respectively, from wk 8 to 32 of randomized treatment and only 15% of pts achieved CHR at wk 32. Following crossover to RUX, 62% of pts achieved spleen response and PBT requirements decreased (64% of pts did not require any PBT and no pt required ≥2 PBT from wk 32 to 80 after switching to RUX). A majority of pts randomized to IFN had modest reductions in WBC, PLTs, and JAK2V617F allele burden over the course of treatment (figure), with most pts showing further reduction after crossover to RUX. The rates and incidence of hematologic abnormalities (WBC, PLT, and neutrophils) reduced after crossover to RUX with the exception of hemoglobin (prior to crossover: grade 1 [N=2] and grade 2 [N=1]; after crossover: grade 1 [N=5] and grade 2 [N=2]). Similarly, rates and incidence of nonhematologic AEs (systemic, metabolic, gout, pruritus, and fatigue) reduced after crossover to RUX with the exception of infections (primarily grade 1 or 2). Four pts discontinued crossover treatment due to AE (N=3) or pt decision (N=1). One death due to CNS hemorrhage occurred after crossover to RUX (not related to study drug).
Conclusion
In the HU-resistant/intolerant PV population of the RESPONSE study, no pt treated with IFN achieved spleen response and a limited number of pts achieved HCT control. However, following crossover to RUX, pts showed improvement in hematologic and spleen response with an overall reduction in PBT procedures and a majority of pts being able to achieve spleen response.

Session topic: E-poster
Keyword(s): Interferon, Myeloproliferative disorder, Polycythemia vera, Ruxolitinib
Type: Eposter Presentation
Background
Polycythemia vera (PV) is characterized by excessive proliferation of erythroid, myeloid, and megakaryocytic components in the bone resulting in an increased risk of thromboembolic events, cardiovascular complications, and disease progression. Both hydroxyurea (HU) and interferon-alpha (IFN) are used as first-line treatment for high-risk pts with PV but pts may become resistant/intolerant (Barbui. JCO 2011; 29(6):761-770). The recent phase 3 RESPONSE study showed that ruxolitinib (RUX) was superior to best available therapy (BAT) following HU resistance/intolerance; however, little information has been presented on the sub-group of pts treated with IFN in BAT arm.
Aims
This subgroup analysis of RESPONSE study evaluates the safety and efficacy of pts treated with IFN in the randomized BAT arm before and after crossover to RUX.
Methods
RESPONSE, an open-label, phase 3 study enrolled pts with PV, who were resistant to or intolerant of HU per modified European LeukemiaNet criteria, had splenomegaly and required phlebotomy (PBT) to control hematocrit (HCT). Pts were randomized 1:1 to RUX (110 pts) 10 mg bid or BAT (112 pts), of which 13 pts received at least one dose of IFN during the randomized treatment phase. Pts were evaluated for HCT control (<45%) without PBT; spleen response (≥35% reduction in spleen volume from baseline by magnetic resonance imaging); and complete hematologic response [CHR: HCT<45%, white blood cell (WBC) ≤10 × 109/L, platelet (PLT) count ≤400 x 109/L]. JAK2V617F allele burden, adverse events (AEs) and hematologic abnormalities were assessed for randomized and crossover treatment.
Results
Baseline demographics were comparable among RUX (N=110) and IFN-treated pts of the BAT arm (N=13). Three pts (23%) received IFN at some time prior to inclusion in the RESPONSE trial. On trial, 9 pts were treated with PEG-IFN and 4 pts were treated with non-PEG-IFN at varying doses. All IFN pts discontinued and crossed over to RUX due to lack of efficacy (11 pts crossed over prior to wk 48 and 2 pts after wk 48). No pt treated with IFN achieved spleen response and only 23% of pts achieved HCT control without PBT from wk 8 to 32 (40% and 60% while on RUX therapy, respectively). Thirty-eight percent, 31%, and 31% of pts required no PBT, 1 PBT, and ≥2 PBT, respectively, from wk 8 to 32 of randomized treatment and only 15% of pts achieved CHR at wk 32. Following crossover to RUX, 62% of pts achieved spleen response and PBT requirements decreased (64% of pts did not require any PBT and no pt required ≥2 PBT from wk 32 to 80 after switching to RUX). A majority of pts randomized to IFN had modest reductions in WBC, PLTs, and JAK2V617F allele burden over the course of treatment (figure), with most pts showing further reduction after crossover to RUX. The rates and incidence of hematologic abnormalities (WBC, PLT, and neutrophils) reduced after crossover to RUX with the exception of hemoglobin (prior to crossover: grade 1 [N=2] and grade 2 [N=1]; after crossover: grade 1 [N=5] and grade 2 [N=2]). Similarly, rates and incidence of nonhematologic AEs (systemic, metabolic, gout, pruritus, and fatigue) reduced after crossover to RUX with the exception of infections (primarily grade 1 or 2). Four pts discontinued crossover treatment due to AE (N=3) or pt decision (N=1). One death due to CNS hemorrhage occurred after crossover to RUX (not related to study drug).
Conclusion
In the HU-resistant/intolerant PV population of the RESPONSE study, no pt treated with IFN achieved spleen response and a limited number of pts achieved HCT control. However, following crossover to RUX, pts showed improvement in hematologic and spleen response with an overall reduction in PBT procedures and a majority of pts being able to achieve spleen response.

Session topic: E-poster
Keyword(s): Interferon, Myeloproliferative disorder, Polycythemia vera, Ruxolitinib
{{ help_message }}
{{filter}}


