A PHASE 1 OPEN LABEL STUDY TO DETERMINE THE PHARMACOKINETICS OF PACRITINIB IN PATIENTS WITH MILD TO SEVERE RENAL IMPAIRMENT AND END STAGE RENAL DISEASE (ESRD) COMPARED WITH HEALTHY SUBJECTS
(Abstract release date: 05/19/16)
EHA Library. Al-Fayoumi S. 06/09/16; 132908; E1359

Dr. Suliman Al-Fayoumi
Contributions
Contributions
Abstract
Abstract: E1359
Type: Eposter Presentation
Background
Treatment and management of patients with hematologic malignancies and co-morbid renal impairment (RI) is challenging as the kidneys are a major route of excretion for many therapeutic agents. The conduct of a RI study is an important regulatory requirement to support appropriate dosage recommendation in patients with RI. Patients with myelofibrosis (MF) and moderate to severe RI treated with the JAK1/JAK2 inhibitor ruxolitinib require dosage reductions. Pacritinib is an oral kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CSF1R being evaluated for patients with primary or secondary MF.
Aims
Evaluate the pharmacokinetics (PK) and safety of pacritinib and its major metabolite in patients with RI (mild, moderate, severe, and ESRD requiring hemodialysis) compared with healthy age-, sex-, and weight-matched control participants.
Methods
Study participants (N=39) who provided written informed consent included healthy volunteers and subjects with RI (mild, moderate, severe based on estimated glomerular filtration rate and ESRD on hemodialysis). All participants except those with ESRD received a single dose of pacritinib 400 mg, with PK and urine assessments conducted for up to 168 h post-dose. Subjects with ESRD received 1 dose of pacritinib 400 mg in each of 2 periods (dialysis and inter-dialysis) separated by a 14-day washout. Pacritinib was administered immediately before and after hemodialysis in the dialysis and inter-dialysis periods, respectively, followed by PK assessments for 72 h post-dose.
Results
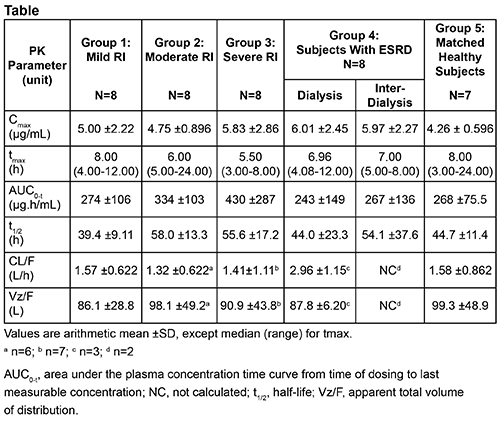
Time to maximum plasma concentration (tmax) was reached at 8, 6, and 5.5 h post-dose in patients with mild, moderate, and severe RI, respectively; tmax was 7 h in patients with ESRD. In healthy controls, tmax was 8 h post-dose. Pacritinib plasma concentrations demonstrated comparable elimination phases across RI groups. Excretion of pacritinib in urine was minimal (1.0-1.4% of dose over 72 h interval) and RI did not decrease urinary excretion. PK analysis revealed slightly higher maximum plasma concentration (Cmax) and total exposure (area under the plasma concentration-time curve [AUC]) in patients with RI vs healthy controls, which are unlikely to be of clinical relevance (Table). Cmax and AUC were similar for patients with ESRD during dialysis and inter-dialysis periods. Apparent total clearance (CL/F) was comparable across RI groups. All adverse events (AEs) were grade 1-2; diarrhea was the most frequent AE.
Conclusion
PK parameters and renal excretion of pacritinib were not significantly affected by RI. Administration of 400 mg single doses of pacritinib to patients with RI was well tolerated. Dosage adjustment for pacritinib is not warranted in patients with RI.
Session topic: E-poster
Keyword(s): Myelofibrosis, Pharmacokinetic, Phase I, Renal impairment
Type: Eposter Presentation
Background
Treatment and management of patients with hematologic malignancies and co-morbid renal impairment (RI) is challenging as the kidneys are a major route of excretion for many therapeutic agents. The conduct of a RI study is an important regulatory requirement to support appropriate dosage recommendation in patients with RI. Patients with myelofibrosis (MF) and moderate to severe RI treated with the JAK1/JAK2 inhibitor ruxolitinib require dosage reductions. Pacritinib is an oral kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CSF1R being evaluated for patients with primary or secondary MF.
Aims
Evaluate the pharmacokinetics (PK) and safety of pacritinib and its major metabolite in patients with RI (mild, moderate, severe, and ESRD requiring hemodialysis) compared with healthy age-, sex-, and weight-matched control participants.
Methods
Study participants (N=39) who provided written informed consent included healthy volunteers and subjects with RI (mild, moderate, severe based on estimated glomerular filtration rate and ESRD on hemodialysis). All participants except those with ESRD received a single dose of pacritinib 400 mg, with PK and urine assessments conducted for up to 168 h post-dose. Subjects with ESRD received 1 dose of pacritinib 400 mg in each of 2 periods (dialysis and inter-dialysis) separated by a 14-day washout. Pacritinib was administered immediately before and after hemodialysis in the dialysis and inter-dialysis periods, respectively, followed by PK assessments for 72 h post-dose.
Results
Time to maximum plasma concentration (tmax) was reached at 8, 6, and 5.5 h post-dose in patients with mild, moderate, and severe RI, respectively; tmax was 7 h in patients with ESRD. In healthy controls, tmax was 8 h post-dose. Pacritinib plasma concentrations demonstrated comparable elimination phases across RI groups. Excretion of pacritinib in urine was minimal (1.0-1.4% of dose over 72 h interval) and RI did not decrease urinary excretion. PK analysis revealed slightly higher maximum plasma concentration (Cmax) and total exposure (area under the plasma concentration-time curve [AUC]) in patients with RI vs healthy controls, which are unlikely to be of clinical relevance (Table). Cmax and AUC were similar for patients with ESRD during dialysis and inter-dialysis periods. Apparent total clearance (CL/F) was comparable across RI groups. All adverse events (AEs) were grade 1-2; diarrhea was the most frequent AE.
Conclusion
PK parameters and renal excretion of pacritinib were not significantly affected by RI. Administration of 400 mg single doses of pacritinib to patients with RI was well tolerated. Dosage adjustment for pacritinib is not warranted in patients with RI.

Session topic: E-poster
Keyword(s): Myelofibrosis, Pharmacokinetic, Phase I, Renal impairment
Abstract: E1359
Type: Eposter Presentation
Background
Treatment and management of patients with hematologic malignancies and co-morbid renal impairment (RI) is challenging as the kidneys are a major route of excretion for many therapeutic agents. The conduct of a RI study is an important regulatory requirement to support appropriate dosage recommendation in patients with RI. Patients with myelofibrosis (MF) and moderate to severe RI treated with the JAK1/JAK2 inhibitor ruxolitinib require dosage reductions. Pacritinib is an oral kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CSF1R being evaluated for patients with primary or secondary MF.
Aims
Evaluate the pharmacokinetics (PK) and safety of pacritinib and its major metabolite in patients with RI (mild, moderate, severe, and ESRD requiring hemodialysis) compared with healthy age-, sex-, and weight-matched control participants.
Methods
Study participants (N=39) who provided written informed consent included healthy volunteers and subjects with RI (mild, moderate, severe based on estimated glomerular filtration rate and ESRD on hemodialysis). All participants except those with ESRD received a single dose of pacritinib 400 mg, with PK and urine assessments conducted for up to 168 h post-dose. Subjects with ESRD received 1 dose of pacritinib 400 mg in each of 2 periods (dialysis and inter-dialysis) separated by a 14-day washout. Pacritinib was administered immediately before and after hemodialysis in the dialysis and inter-dialysis periods, respectively, followed by PK assessments for 72 h post-dose.
Results
Time to maximum plasma concentration (tmax) was reached at 8, 6, and 5.5 h post-dose in patients with mild, moderate, and severe RI, respectively; tmax was 7 h in patients with ESRD. In healthy controls, tmax was 8 h post-dose. Pacritinib plasma concentrations demonstrated comparable elimination phases across RI groups. Excretion of pacritinib in urine was minimal (1.0-1.4% of dose over 72 h interval) and RI did not decrease urinary excretion. PK analysis revealed slightly higher maximum plasma concentration (Cmax) and total exposure (area under the plasma concentration-time curve [AUC]) in patients with RI vs healthy controls, which are unlikely to be of clinical relevance (Table). Cmax and AUC were similar for patients with ESRD during dialysis and inter-dialysis periods. Apparent total clearance (CL/F) was comparable across RI groups. All adverse events (AEs) were grade 1-2; diarrhea was the most frequent AE.
Conclusion
PK parameters and renal excretion of pacritinib were not significantly affected by RI. Administration of 400 mg single doses of pacritinib to patients with RI was well tolerated. Dosage adjustment for pacritinib is not warranted in patients with RI.

Session topic: E-poster
Keyword(s): Myelofibrosis, Pharmacokinetic, Phase I, Renal impairment
Type: Eposter Presentation
Background
Treatment and management of patients with hematologic malignancies and co-morbid renal impairment (RI) is challenging as the kidneys are a major route of excretion for many therapeutic agents. The conduct of a RI study is an important regulatory requirement to support appropriate dosage recommendation in patients with RI. Patients with myelofibrosis (MF) and moderate to severe RI treated with the JAK1/JAK2 inhibitor ruxolitinib require dosage reductions. Pacritinib is an oral kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CSF1R being evaluated for patients with primary or secondary MF.
Aims
Evaluate the pharmacokinetics (PK) and safety of pacritinib and its major metabolite in patients with RI (mild, moderate, severe, and ESRD requiring hemodialysis) compared with healthy age-, sex-, and weight-matched control participants.
Methods
Study participants (N=39) who provided written informed consent included healthy volunteers and subjects with RI (mild, moderate, severe based on estimated glomerular filtration rate and ESRD on hemodialysis). All participants except those with ESRD received a single dose of pacritinib 400 mg, with PK and urine assessments conducted for up to 168 h post-dose. Subjects with ESRD received 1 dose of pacritinib 400 mg in each of 2 periods (dialysis and inter-dialysis) separated by a 14-day washout. Pacritinib was administered immediately before and after hemodialysis in the dialysis and inter-dialysis periods, respectively, followed by PK assessments for 72 h post-dose.
Results
Time to maximum plasma concentration (tmax) was reached at 8, 6, and 5.5 h post-dose in patients with mild, moderate, and severe RI, respectively; tmax was 7 h in patients with ESRD. In healthy controls, tmax was 8 h post-dose. Pacritinib plasma concentrations demonstrated comparable elimination phases across RI groups. Excretion of pacritinib in urine was minimal (1.0-1.4% of dose over 72 h interval) and RI did not decrease urinary excretion. PK analysis revealed slightly higher maximum plasma concentration (Cmax) and total exposure (area under the plasma concentration-time curve [AUC]) in patients with RI vs healthy controls, which are unlikely to be of clinical relevance (Table). Cmax and AUC were similar for patients with ESRD during dialysis and inter-dialysis periods. Apparent total clearance (CL/F) was comparable across RI groups. All adverse events (AEs) were grade 1-2; diarrhea was the most frequent AE.
Conclusion
PK parameters and renal excretion of pacritinib were not significantly affected by RI. Administration of 400 mg single doses of pacritinib to patients with RI was well tolerated. Dosage adjustment for pacritinib is not warranted in patients with RI.

Session topic: E-poster
Keyword(s): Myelofibrosis, Pharmacokinetic, Phase I, Renal impairment
{{ help_message }}
{{filter}}


