MORPHOLOGIC DYSPLASIA IS ASSOCIATED WITH A MORE AGGRESSIVE DISEASE PHENOTYPE IN MYELOFIBROSIS
(Abstract release date: 05/19/16)
EHA Library. Byrne M. 06/09/16; 132902; E1353

Dr. Michael Byrne
Contributions
Contributions
Abstract
Abstract: E1353
Type: Eposter Presentation
Background
Primary myelofibrosis (PMF), post-essential thrombocytemia-MF (PET-MF) and post-polycythemia vera-MF (PPV-MF) are Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs). These diseases are frequently associated with driver mutations within the JAK-STAT signaling cascade. However, additional mutations may be acquired during disease progression. MDS/MPNs are distinct myeloid diseases with similar genetic and morphologic characteristics of myelodysplasia and MPNs. To better understand the significance of these mutations, we correlated DNA mutation profiles with bone marrow (BM) dysplasia and clinical outcomes.
Aims
Evaluate the impact of non-driver mutations and BM dysplasia on clinical outcomes in patients with PET-MF, PPV-MF, and PMF.
Methods
We identified 19 patients diagnosed with PMF and 18 patients with PET- or PPV-MF, reviewed their BM biopsies and aspirates, and performed NGS with a 37 gene myeloid panel. At least 2 hematopathologists, blinded to original diagnosis and genetic results, classified the BM biopsies according to WHO guidelines and morphologic dysplasia. A committee of hematologists and hematopathologists reviewed all data and rendered a consensus diagnosis. Statistical comparisons between groups relied on the two-sided student’s t-test; the log-rank test was utilized for survival assessments. This study was approved by our IRB
Results
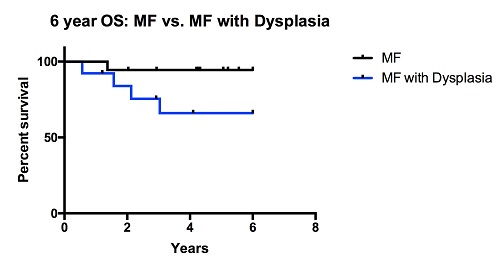
The mean age at diagnosis was 57.1 years and NGS occurred at a median of 2.2 months from diagnosis; the mean length of follow-up is 8.2 years. Fourteen patients (39%) had an abnormal karyotype. JAK2 mutation V617F was identified in 25 (68%) patients, CALR or MPL mutations were present in 8 (22%), and 4 had no identifiable driver mutation. Patients had a mean of 2 mutations; TET2, EZH2, IDH1, IDH2, DNMT3A, and ASXL1 represent the most common mutations. For analysis, patients were divided according to the dynamic international prognostic scoring system plus risk: high (7), int-2 (17), and low/int-1 risk (13) disease. Those with high-risk disease were significantly more likely to have BM dysplasia relative to those with low/int-1 disease (77% vs. 33%, P: <0.05) and twice as likely to be re-categorized as MDS/MPN overlap syndrome (67% vs. 35% and 23%) during consensus review. These patients had a trend toward a higher mutational burden (3* vs. 2.2 and 1.7* mutations, *P: 0.09), and were twice as likely to have an unfavorable karyotype (*67% vs. 41% and *8%, *P: <0.05). Clinically, these patients experienced a more aggressive disease phenotype with a shortened overall survival (OS) compared to patients with non-dysplastic MF. Five patients in this analysis had TET2 mutations. TET2 mutated patients were more likely to have BM dysplasia compared to wild type (WT) (80% vs. 50%). These patients were younger at diagnosis (mean: 49.3 vs. 60.4 years, P: <0.05), had a higher number of non-driver mutations (3.8 vs. 1.2, P: 0.001), were more likely to have an unfavorable karyotype (80% vs. 42%) and circulating blasts (40% vs. 14%). Six year OS was significantly shorter in the TET2 mutated group vs. WT (P: <0.05).
Conclusion
Distinguishing between ‘MF with dysplasia’ and MDS/MPN is a diagnostic challenge because the significance and natural history of MPN dysplasia is poorly understood. Our data demonstrate that this dysplasia is associated with established clinical and genetic markers of aggressive disease, including mutations of TET2. Taken together, this suggests that MPN with dysplasia represents a MPN with aggressive disease biology and shortened survival.
Session topic: E-poster
Type: Eposter Presentation
Background
Primary myelofibrosis (PMF), post-essential thrombocytemia-MF (PET-MF) and post-polycythemia vera-MF (PPV-MF) are Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs). These diseases are frequently associated with driver mutations within the JAK-STAT signaling cascade. However, additional mutations may be acquired during disease progression. MDS/MPNs are distinct myeloid diseases with similar genetic and morphologic characteristics of myelodysplasia and MPNs. To better understand the significance of these mutations, we correlated DNA mutation profiles with bone marrow (BM) dysplasia and clinical outcomes.
Aims
Evaluate the impact of non-driver mutations and BM dysplasia on clinical outcomes in patients with PET-MF, PPV-MF, and PMF.
Methods
We identified 19 patients diagnosed with PMF and 18 patients with PET- or PPV-MF, reviewed their BM biopsies and aspirates, and performed NGS with a 37 gene myeloid panel. At least 2 hematopathologists, blinded to original diagnosis and genetic results, classified the BM biopsies according to WHO guidelines and morphologic dysplasia. A committee of hematologists and hematopathologists reviewed all data and rendered a consensus diagnosis. Statistical comparisons between groups relied on the two-sided student’s t-test; the log-rank test was utilized for survival assessments. This study was approved by our IRB
Results
The mean age at diagnosis was 57.1 years and NGS occurred at a median of 2.2 months from diagnosis; the mean length of follow-up is 8.2 years. Fourteen patients (39%) had an abnormal karyotype. JAK2 mutation V617F was identified in 25 (68%) patients, CALR or MPL mutations were present in 8 (22%), and 4 had no identifiable driver mutation. Patients had a mean of 2 mutations; TET2, EZH2, IDH1, IDH2, DNMT3A, and ASXL1 represent the most common mutations. For analysis, patients were divided according to the dynamic international prognostic scoring system plus risk: high (7), int-2 (17), and low/int-1 risk (13) disease. Those with high-risk disease were significantly more likely to have BM dysplasia relative to those with low/int-1 disease (77% vs. 33%, P: <0.05) and twice as likely to be re-categorized as MDS/MPN overlap syndrome (67% vs. 35% and 23%) during consensus review. These patients had a trend toward a higher mutational burden (3* vs. 2.2 and 1.7* mutations, *P: 0.09), and were twice as likely to have an unfavorable karyotype (*67% vs. 41% and *8%, *P: <0.05). Clinically, these patients experienced a more aggressive disease phenotype with a shortened overall survival (OS) compared to patients with non-dysplastic MF. Five patients in this analysis had TET2 mutations. TET2 mutated patients were more likely to have BM dysplasia compared to wild type (WT) (80% vs. 50%). These patients were younger at diagnosis (mean: 49.3 vs. 60.4 years, P: <0.05), had a higher number of non-driver mutations (3.8 vs. 1.2, P: 0.001), were more likely to have an unfavorable karyotype (80% vs. 42%) and circulating blasts (40% vs. 14%). Six year OS was significantly shorter in the TET2 mutated group vs. WT (P: <0.05).
Conclusion
Distinguishing between ‘MF with dysplasia’ and MDS/MPN is a diagnostic challenge because the significance and natural history of MPN dysplasia is poorly understood. Our data demonstrate that this dysplasia is associated with established clinical and genetic markers of aggressive disease, including mutations of TET2. Taken together, this suggests that MPN with dysplasia represents a MPN with aggressive disease biology and shortened survival.

Session topic: E-poster
Abstract: E1353
Type: Eposter Presentation
Background
Primary myelofibrosis (PMF), post-essential thrombocytemia-MF (PET-MF) and post-polycythemia vera-MF (PPV-MF) are Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs). These diseases are frequently associated with driver mutations within the JAK-STAT signaling cascade. However, additional mutations may be acquired during disease progression. MDS/MPNs are distinct myeloid diseases with similar genetic and morphologic characteristics of myelodysplasia and MPNs. To better understand the significance of these mutations, we correlated DNA mutation profiles with bone marrow (BM) dysplasia and clinical outcomes.
Aims
Evaluate the impact of non-driver mutations and BM dysplasia on clinical outcomes in patients with PET-MF, PPV-MF, and PMF.
Methods
We identified 19 patients diagnosed with PMF and 18 patients with PET- or PPV-MF, reviewed their BM biopsies and aspirates, and performed NGS with a 37 gene myeloid panel. At least 2 hematopathologists, blinded to original diagnosis and genetic results, classified the BM biopsies according to WHO guidelines and morphologic dysplasia. A committee of hematologists and hematopathologists reviewed all data and rendered a consensus diagnosis. Statistical comparisons between groups relied on the two-sided student’s t-test; the log-rank test was utilized for survival assessments. This study was approved by our IRB
Results
The mean age at diagnosis was 57.1 years and NGS occurred at a median of 2.2 months from diagnosis; the mean length of follow-up is 8.2 years. Fourteen patients (39%) had an abnormal karyotype. JAK2 mutation V617F was identified in 25 (68%) patients, CALR or MPL mutations were present in 8 (22%), and 4 had no identifiable driver mutation. Patients had a mean of 2 mutations; TET2, EZH2, IDH1, IDH2, DNMT3A, and ASXL1 represent the most common mutations. For analysis, patients were divided according to the dynamic international prognostic scoring system plus risk: high (7), int-2 (17), and low/int-1 risk (13) disease. Those with high-risk disease were significantly more likely to have BM dysplasia relative to those with low/int-1 disease (77% vs. 33%, P: <0.05) and twice as likely to be re-categorized as MDS/MPN overlap syndrome (67% vs. 35% and 23%) during consensus review. These patients had a trend toward a higher mutational burden (3* vs. 2.2 and 1.7* mutations, *P: 0.09), and were twice as likely to have an unfavorable karyotype (*67% vs. 41% and *8%, *P: <0.05). Clinically, these patients experienced a more aggressive disease phenotype with a shortened overall survival (OS) compared to patients with non-dysplastic MF. Five patients in this analysis had TET2 mutations. TET2 mutated patients were more likely to have BM dysplasia compared to wild type (WT) (80% vs. 50%). These patients were younger at diagnosis (mean: 49.3 vs. 60.4 years, P: <0.05), had a higher number of non-driver mutations (3.8 vs. 1.2, P: 0.001), were more likely to have an unfavorable karyotype (80% vs. 42%) and circulating blasts (40% vs. 14%). Six year OS was significantly shorter in the TET2 mutated group vs. WT (P: <0.05).
Conclusion
Distinguishing between ‘MF with dysplasia’ and MDS/MPN is a diagnostic challenge because the significance and natural history of MPN dysplasia is poorly understood. Our data demonstrate that this dysplasia is associated with established clinical and genetic markers of aggressive disease, including mutations of TET2. Taken together, this suggests that MPN with dysplasia represents a MPN with aggressive disease biology and shortened survival.

Session topic: E-poster
Type: Eposter Presentation
Background
Primary myelofibrosis (PMF), post-essential thrombocytemia-MF (PET-MF) and post-polycythemia vera-MF (PPV-MF) are Philadelphia-chromosome negative myeloproliferative neoplasms (MPNs). These diseases are frequently associated with driver mutations within the JAK-STAT signaling cascade. However, additional mutations may be acquired during disease progression. MDS/MPNs are distinct myeloid diseases with similar genetic and morphologic characteristics of myelodysplasia and MPNs. To better understand the significance of these mutations, we correlated DNA mutation profiles with bone marrow (BM) dysplasia and clinical outcomes.
Aims
Evaluate the impact of non-driver mutations and BM dysplasia on clinical outcomes in patients with PET-MF, PPV-MF, and PMF.
Methods
We identified 19 patients diagnosed with PMF and 18 patients with PET- or PPV-MF, reviewed their BM biopsies and aspirates, and performed NGS with a 37 gene myeloid panel. At least 2 hematopathologists, blinded to original diagnosis and genetic results, classified the BM biopsies according to WHO guidelines and morphologic dysplasia. A committee of hematologists and hematopathologists reviewed all data and rendered a consensus diagnosis. Statistical comparisons between groups relied on the two-sided student’s t-test; the log-rank test was utilized for survival assessments. This study was approved by our IRB
Results
The mean age at diagnosis was 57.1 years and NGS occurred at a median of 2.2 months from diagnosis; the mean length of follow-up is 8.2 years. Fourteen patients (39%) had an abnormal karyotype. JAK2 mutation V617F was identified in 25 (68%) patients, CALR or MPL mutations were present in 8 (22%), and 4 had no identifiable driver mutation. Patients had a mean of 2 mutations; TET2, EZH2, IDH1, IDH2, DNMT3A, and ASXL1 represent the most common mutations. For analysis, patients were divided according to the dynamic international prognostic scoring system plus risk: high (7), int-2 (17), and low/int-1 risk (13) disease. Those with high-risk disease were significantly more likely to have BM dysplasia relative to those with low/int-1 disease (77% vs. 33%, P: <0.05) and twice as likely to be re-categorized as MDS/MPN overlap syndrome (67% vs. 35% and 23%) during consensus review. These patients had a trend toward a higher mutational burden (3* vs. 2.2 and 1.7* mutations, *P: 0.09), and were twice as likely to have an unfavorable karyotype (*67% vs. 41% and *8%, *P: <0.05). Clinically, these patients experienced a more aggressive disease phenotype with a shortened overall survival (OS) compared to patients with non-dysplastic MF. Five patients in this analysis had TET2 mutations. TET2 mutated patients were more likely to have BM dysplasia compared to wild type (WT) (80% vs. 50%). These patients were younger at diagnosis (mean: 49.3 vs. 60.4 years, P: <0.05), had a higher number of non-driver mutations (3.8 vs. 1.2, P: 0.001), were more likely to have an unfavorable karyotype (80% vs. 42%) and circulating blasts (40% vs. 14%). Six year OS was significantly shorter in the TET2 mutated group vs. WT (P: <0.05).
Conclusion
Distinguishing between ‘MF with dysplasia’ and MDS/MPN is a diagnostic challenge because the significance and natural history of MPN dysplasia is poorly understood. Our data demonstrate that this dysplasia is associated with established clinical and genetic markers of aggressive disease, including mutations of TET2. Taken together, this suggests that MPN with dysplasia represents a MPN with aggressive disease biology and shortened survival.

Session topic: E-poster
{{ help_message }}
{{filter}}


