PALPITATIONS AND ARRHYTHMIA IN 3649 HIGH-RISK PATIENTS WITH ESSENTIAL THROMBOCYTHEMIA: RESULTS FROM THE PROSPECTIVE LONG-TERM OBSERVATIONAL EXELS STUDY
(Abstract release date: 05/19/16)
EHA Library. Gugliotta L. 06/09/16; 132893; E1344

Dr. Luigi Gugliotta
Contributions
Contributions
Abstract
Abstract: E1344
Type: Eposter Presentation
Background
The Evaluation of Xagrid Efficacy and Long-term Safety (EXELS) study (NCT00567502) is the largest prospective observational cohort of high-risk patients with essential thrombocythemia (ET) reported to date. Although anagrelide (ANA) is typically well tolerated, as patients with ET often have cardiac risk factors, it is important to analyze ANA’s cardiac safety in these patients.
Aims
The primary objective of EXELS was safety and pregnancy outcomes of ANA compared with other cytoreductive therapies (CRT). Here we assess palpitations and arrhythmia events as reported in EXELS.
Methods
High-risk patients (≥1 of age >60 years, previous thrombotic event, platelet count >1000x109/L) with ET were enrolled across 13 countries in Europe from 2005–2009. Patients were required to be receiving CRT. Data, including events predefined in the protocol (PDEs), were collected every 6 months for 5 years for all patients.
Results
3649 patients were categorized according to treatment at registration as follows: ANA (n=804), ANA + other CRT (n=141), other CRT (n=2666). Over 80% of patients received either hydroxycarbamide (HC) or ANA, and 69.8% of patients received anti-aggregatory therapy. At registration, median age was lower in the ANA (55.5 years) and ANA + other CRT (59.0 years) groups vs the other CRT group (70.0 years).
Due to the non-interventional nature of the study, patients were able to switch treatments and did not necessarily remain in the same group over the entire observation period. As such, the analysis of palpitations and arrhythmia events were performed according to the treatment group patients were on for at least one day during the study.
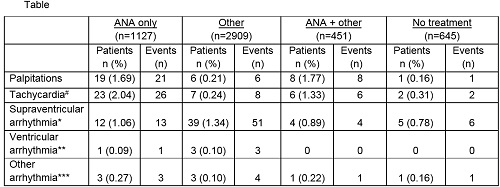
Higher rates of palpitations were observed in patients receiving ANA alone (1.69%, n=19) vs other CRT alone (0.21%, n=6; Table). Similar differences were observed for tachycardia# events; 2.04% of patients (n=23) receiving ANA experienced tachycardia compared to 0.24% (n=7) of those receiving other CRT.
There did not appear to be a clear difference in rates of supraventricular arrhythmia* events observed between patients receiving ANA (1.06%, n=12) or other CRT (1.34%, n=39). This was also observed with ventricular arrhythmias** with no apparent difference between the ANA only group (0.09%, n=1) vs other CRT (0.10%, n=3). Very low rates of other arrhythmias*** were observed across all groups.
Similar rates of palpitations, tachycardia and arrhythmias (supraventricular, ventricular and other) were seen in patients receiving ANA + other CRT compared with ANA alone.
Conclusion
Of the most commonly reported cardiac PDEs, palpitations and tachycardia were more frequently observed with ANA vs other CRT. Arrhythmia events (supraventricular, ventricular and other) were observed at similar rates in the ANA and other CRT groups.
Palpitations, tachycardia and arrhythmia events were reported at a similar rate in the ANA + other CRT group compared with the ANA group. While there may be an increase in specific cardiac events with ANA, these are mostly mild and reversible with appropriate treatment.
Table footnotes:#Tachycardia comprises tachycardia, tachycardia paroxysmal and tachyarrhythmia (not otherwise defined).
*Supraventricular arrhythmia comprises atrial fibrillation/atrial flutter, arrhythmia supraventricular, supraventricular extrasystoles, supraventricular tachycardia and sinus tachycardia.
**Ventricular arrhythmia comprises ventricular fibrillation/ventricular tachycardia/ventricular extrasystoles.
***Other arrhythmia comprises arrhythmia (not otherwise defined), bradycardia, extrasystoles, sinus bradycardia.
Session topic: E-poster
Keyword(s): Anagrelide, Essential Thrombocytemia, Safety
Type: Eposter Presentation
Background
The Evaluation of Xagrid Efficacy and Long-term Safety (EXELS) study (NCT00567502) is the largest prospective observational cohort of high-risk patients with essential thrombocythemia (ET) reported to date. Although anagrelide (ANA) is typically well tolerated, as patients with ET often have cardiac risk factors, it is important to analyze ANA’s cardiac safety in these patients.
Aims
The primary objective of EXELS was safety and pregnancy outcomes of ANA compared with other cytoreductive therapies (CRT). Here we assess palpitations and arrhythmia events as reported in EXELS.
Methods
High-risk patients (≥1 of age >60 years, previous thrombotic event, platelet count >1000x109/L) with ET were enrolled across 13 countries in Europe from 2005–2009. Patients were required to be receiving CRT. Data, including events predefined in the protocol (PDEs), were collected every 6 months for 5 years for all patients.
Results
3649 patients were categorized according to treatment at registration as follows: ANA (n=804), ANA + other CRT (n=141), other CRT (n=2666). Over 80% of patients received either hydroxycarbamide (HC) or ANA, and 69.8% of patients received anti-aggregatory therapy. At registration, median age was lower in the ANA (55.5 years) and ANA + other CRT (59.0 years) groups vs the other CRT group (70.0 years).
Due to the non-interventional nature of the study, patients were able to switch treatments and did not necessarily remain in the same group over the entire observation period. As such, the analysis of palpitations and arrhythmia events were performed according to the treatment group patients were on for at least one day during the study.
Higher rates of palpitations were observed in patients receiving ANA alone (1.69%, n=19) vs other CRT alone (0.21%, n=6; Table). Similar differences were observed for tachycardia# events; 2.04% of patients (n=23) receiving ANA experienced tachycardia compared to 0.24% (n=7) of those receiving other CRT.
There did not appear to be a clear difference in rates of supraventricular arrhythmia* events observed between patients receiving ANA (1.06%, n=12) or other CRT (1.34%, n=39). This was also observed with ventricular arrhythmias** with no apparent difference between the ANA only group (0.09%, n=1) vs other CRT (0.10%, n=3). Very low rates of other arrhythmias*** were observed across all groups.
Similar rates of palpitations, tachycardia and arrhythmias (supraventricular, ventricular and other) were seen in patients receiving ANA + other CRT compared with ANA alone.
Conclusion
Of the most commonly reported cardiac PDEs, palpitations and tachycardia were more frequently observed with ANA vs other CRT. Arrhythmia events (supraventricular, ventricular and other) were observed at similar rates in the ANA and other CRT groups.
Palpitations, tachycardia and arrhythmia events were reported at a similar rate in the ANA + other CRT group compared with the ANA group. While there may be an increase in specific cardiac events with ANA, these are mostly mild and reversible with appropriate treatment.
Table footnotes:#Tachycardia comprises tachycardia, tachycardia paroxysmal and tachyarrhythmia (not otherwise defined).
*Supraventricular arrhythmia comprises atrial fibrillation/atrial flutter, arrhythmia supraventricular, supraventricular extrasystoles, supraventricular tachycardia and sinus tachycardia.
**Ventricular arrhythmia comprises ventricular fibrillation/ventricular tachycardia/ventricular extrasystoles.
***Other arrhythmia comprises arrhythmia (not otherwise defined), bradycardia, extrasystoles, sinus bradycardia.

Session topic: E-poster
Keyword(s): Anagrelide, Essential Thrombocytemia, Safety
Abstract: E1344
Type: Eposter Presentation
Background
The Evaluation of Xagrid Efficacy and Long-term Safety (EXELS) study (NCT00567502) is the largest prospective observational cohort of high-risk patients with essential thrombocythemia (ET) reported to date. Although anagrelide (ANA) is typically well tolerated, as patients with ET often have cardiac risk factors, it is important to analyze ANA’s cardiac safety in these patients.
Aims
The primary objective of EXELS was safety and pregnancy outcomes of ANA compared with other cytoreductive therapies (CRT). Here we assess palpitations and arrhythmia events as reported in EXELS.
Methods
High-risk patients (≥1 of age >60 years, previous thrombotic event, platelet count >1000x109/L) with ET were enrolled across 13 countries in Europe from 2005–2009. Patients were required to be receiving CRT. Data, including events predefined in the protocol (PDEs), were collected every 6 months for 5 years for all patients.
Results
3649 patients were categorized according to treatment at registration as follows: ANA (n=804), ANA + other CRT (n=141), other CRT (n=2666). Over 80% of patients received either hydroxycarbamide (HC) or ANA, and 69.8% of patients received anti-aggregatory therapy. At registration, median age was lower in the ANA (55.5 years) and ANA + other CRT (59.0 years) groups vs the other CRT group (70.0 years).
Due to the non-interventional nature of the study, patients were able to switch treatments and did not necessarily remain in the same group over the entire observation period. As such, the analysis of palpitations and arrhythmia events were performed according to the treatment group patients were on for at least one day during the study.
Higher rates of palpitations were observed in patients receiving ANA alone (1.69%, n=19) vs other CRT alone (0.21%, n=6; Table). Similar differences were observed for tachycardia# events; 2.04% of patients (n=23) receiving ANA experienced tachycardia compared to 0.24% (n=7) of those receiving other CRT.
There did not appear to be a clear difference in rates of supraventricular arrhythmia* events observed between patients receiving ANA (1.06%, n=12) or other CRT (1.34%, n=39). This was also observed with ventricular arrhythmias** with no apparent difference between the ANA only group (0.09%, n=1) vs other CRT (0.10%, n=3). Very low rates of other arrhythmias*** were observed across all groups.
Similar rates of palpitations, tachycardia and arrhythmias (supraventricular, ventricular and other) were seen in patients receiving ANA + other CRT compared with ANA alone.
Conclusion
Of the most commonly reported cardiac PDEs, palpitations and tachycardia were more frequently observed with ANA vs other CRT. Arrhythmia events (supraventricular, ventricular and other) were observed at similar rates in the ANA and other CRT groups.
Palpitations, tachycardia and arrhythmia events were reported at a similar rate in the ANA + other CRT group compared with the ANA group. While there may be an increase in specific cardiac events with ANA, these are mostly mild and reversible with appropriate treatment.
Table footnotes:#Tachycardia comprises tachycardia, tachycardia paroxysmal and tachyarrhythmia (not otherwise defined).
*Supraventricular arrhythmia comprises atrial fibrillation/atrial flutter, arrhythmia supraventricular, supraventricular extrasystoles, supraventricular tachycardia and sinus tachycardia.
**Ventricular arrhythmia comprises ventricular fibrillation/ventricular tachycardia/ventricular extrasystoles.
***Other arrhythmia comprises arrhythmia (not otherwise defined), bradycardia, extrasystoles, sinus bradycardia.

Session topic: E-poster
Keyword(s): Anagrelide, Essential Thrombocytemia, Safety
Type: Eposter Presentation
Background
The Evaluation of Xagrid Efficacy and Long-term Safety (EXELS) study (NCT00567502) is the largest prospective observational cohort of high-risk patients with essential thrombocythemia (ET) reported to date. Although anagrelide (ANA) is typically well tolerated, as patients with ET often have cardiac risk factors, it is important to analyze ANA’s cardiac safety in these patients.
Aims
The primary objective of EXELS was safety and pregnancy outcomes of ANA compared with other cytoreductive therapies (CRT). Here we assess palpitations and arrhythmia events as reported in EXELS.
Methods
High-risk patients (≥1 of age >60 years, previous thrombotic event, platelet count >1000x109/L) with ET were enrolled across 13 countries in Europe from 2005–2009. Patients were required to be receiving CRT. Data, including events predefined in the protocol (PDEs), were collected every 6 months for 5 years for all patients.
Results
3649 patients were categorized according to treatment at registration as follows: ANA (n=804), ANA + other CRT (n=141), other CRT (n=2666). Over 80% of patients received either hydroxycarbamide (HC) or ANA, and 69.8% of patients received anti-aggregatory therapy. At registration, median age was lower in the ANA (55.5 years) and ANA + other CRT (59.0 years) groups vs the other CRT group (70.0 years).
Due to the non-interventional nature of the study, patients were able to switch treatments and did not necessarily remain in the same group over the entire observation period. As such, the analysis of palpitations and arrhythmia events were performed according to the treatment group patients were on for at least one day during the study.
Higher rates of palpitations were observed in patients receiving ANA alone (1.69%, n=19) vs other CRT alone (0.21%, n=6; Table). Similar differences were observed for tachycardia# events; 2.04% of patients (n=23) receiving ANA experienced tachycardia compared to 0.24% (n=7) of those receiving other CRT.
There did not appear to be a clear difference in rates of supraventricular arrhythmia* events observed between patients receiving ANA (1.06%, n=12) or other CRT (1.34%, n=39). This was also observed with ventricular arrhythmias** with no apparent difference between the ANA only group (0.09%, n=1) vs other CRT (0.10%, n=3). Very low rates of other arrhythmias*** were observed across all groups.
Similar rates of palpitations, tachycardia and arrhythmias (supraventricular, ventricular and other) were seen in patients receiving ANA + other CRT compared with ANA alone.
Conclusion
Of the most commonly reported cardiac PDEs, palpitations and tachycardia were more frequently observed with ANA vs other CRT. Arrhythmia events (supraventricular, ventricular and other) were observed at similar rates in the ANA and other CRT groups.
Palpitations, tachycardia and arrhythmia events were reported at a similar rate in the ANA + other CRT group compared with the ANA group. While there may be an increase in specific cardiac events with ANA, these are mostly mild and reversible with appropriate treatment.
Table footnotes:#Tachycardia comprises tachycardia, tachycardia paroxysmal and tachyarrhythmia (not otherwise defined).
*Supraventricular arrhythmia comprises atrial fibrillation/atrial flutter, arrhythmia supraventricular, supraventricular extrasystoles, supraventricular tachycardia and sinus tachycardia.
**Ventricular arrhythmia comprises ventricular fibrillation/ventricular tachycardia/ventricular extrasystoles.
***Other arrhythmia comprises arrhythmia (not otherwise defined), bradycardia, extrasystoles, sinus bradycardia.

Session topic: E-poster
Keyword(s): Anagrelide, Essential Thrombocytemia, Safety
{{ help_message }}
{{filter}}


