ANALYSIS OF CLINICAL PHENOTYPE AND OUTCOME IN CALR VERSUS JAK2 MUTATED OR TRIPLE-NEGATIVE ESSENTIAL THROMBOCYTEMIA
(Abstract release date: 05/19/16)
EHA Library. Aroldi A. 06/09/16; 132889; E1340

Dr. Andrea Aroldi
Contributions
Contributions
Abstract
Abstract: E1340
Type: Eposter Presentation
Background
About 85% of patients (pts) with Essential Thrombocythemia (ET) harbors one of three driver mutations: JAK2, calreticulin (CALR) and MPL; the remaining are defined 'triple-negative'. Recent studies have analyzed the clinical and hematological features of ET or primary myelofibrosis (MF) according to mutational genotype. However the clinical impact of these mutations seems to differ between cases of ET and MF. In particular controversial data were reported regarding the risk of MF progression in triple-negative and CALR mutated ET.
Aims
We retrospectively analyzed a cohort of 350 ET pts, diagnosed between 1990 and 2015 according to WHO classification, and we compared the clinical phenotypes and outcome of CALR mutant cases with the group of JAK2V617F positive and triple-negative pts.
Methods
Hematological parameters, cardiovascular factors, IPSET score, microvascular symptoms and thrombotic complications were reported for each cohorts of ET pts, as well as cytoreductive treatment. During follow-up, progression to MF and leukemic evolution were also recorded. Mann-Whitney test was used for numerical comparisons while Chi-square test was used for nominal ones. Overall survival (OS) and myelofibrosis-free-survival (MFS) and Leukemia-free-survival (LFS) were estimated by Kaplan–Meier analysis and compared with the Log-rank test.
Results
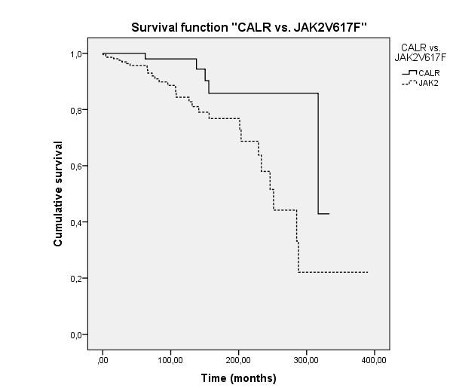
Among 350 ET pts, CALR mutations were detected in 69 (19.7 %), JAK2V617F mutations in 216 (61,7 %) and MPL mutations in 13 (3.7 %). 52 (15%) pts were triple-negative. The median follow-up was 77,5 months, with a longer time of observation for CALR mutants (median 125 months). JAK2-mutated ET had a higher risk of thrombosis at onset, which was about three times higher than CALR mutated pts or triple-negative ET (20,4% vs 7,2% vs 17,3% respectively, p=0,05). No difference between JAK2, CALR mutated and triple-negative ET was identified in terms of incidence of recurrent thrombosis during follow-up. Compared to those with JAK2 mutation, CALR-mutated ET pts were characterized by younger age (p=0,012), lower leukocyte count (p<0,0001) and hematocrit (p<0,002) and higher platelet count (p<0,0001) and presented a lower IPSET score (p<0,0001). Triple-negative pts showed a low IPSET score, similar to CALR-mutated pts; they had a lower incidence of microvascular symptoms (13.5%) in respect to JAK2 (39,8%) and CALR-mutated (34,8%) pts (p<0,0006 and p=0,014, respectively), with a low intensity of cytoreductive treatment. Interestingly, CALR-mutated ET presented an increased risk of progression to MF (24,6%) in comparison with JAK2V617F positive (5,6%) and triple-negative (7,7%) ET (p<0,0001). CALR type 1 deletion seemed to be strongly associated with MF evolution (15/17 pts). Median MFS seemed shorter in JAK2 positive pts (79,77 months) compared to CALR-mutated (106,44 months) and triple-negative ET pts (96,04 months). As shown in Figure 1, we finally documented a better OS in CALR pts than in JAK2 positive group (median OS 317 vs 251 months respectively; p=0,007). No difference in LFS according to different genotypes was demonstrated.
Conclusion
This study in a large cohort of ET supports and extends the growing body of evidence showing that different mutational genotypes in ET present significant differences with regards to clinical and hematologic presentation as well as risk of MF progression. Even though OS was higher, CALR mutation-positive patients appear to have a higher risk of evolution to MF compared to the JAK2 V617F-positive and triple-negative pts.
Session topic: E-poster
Keyword(s): Essential Thrombocytemia, Myelofibrosis, Myeloproliferative disorder
Type: Eposter Presentation
Background
About 85% of patients (pts) with Essential Thrombocythemia (ET) harbors one of three driver mutations: JAK2, calreticulin (CALR) and MPL; the remaining are defined 'triple-negative'. Recent studies have analyzed the clinical and hematological features of ET or primary myelofibrosis (MF) according to mutational genotype. However the clinical impact of these mutations seems to differ between cases of ET and MF. In particular controversial data were reported regarding the risk of MF progression in triple-negative and CALR mutated ET.
Aims
We retrospectively analyzed a cohort of 350 ET pts, diagnosed between 1990 and 2015 according to WHO classification, and we compared the clinical phenotypes and outcome of CALR mutant cases with the group of JAK2V617F positive and triple-negative pts.
Methods
Hematological parameters, cardiovascular factors, IPSET score, microvascular symptoms and thrombotic complications were reported for each cohorts of ET pts, as well as cytoreductive treatment. During follow-up, progression to MF and leukemic evolution were also recorded. Mann-Whitney test was used for numerical comparisons while Chi-square test was used for nominal ones. Overall survival (OS) and myelofibrosis-free-survival (MFS) and Leukemia-free-survival (LFS) were estimated by Kaplan–Meier analysis and compared with the Log-rank test.
Results
Among 350 ET pts, CALR mutations were detected in 69 (19.7 %), JAK2V617F mutations in 216 (61,7 %) and MPL mutations in 13 (3.7 %). 52 (15%) pts were triple-negative. The median follow-up was 77,5 months, with a longer time of observation for CALR mutants (median 125 months). JAK2-mutated ET had a higher risk of thrombosis at onset, which was about three times higher than CALR mutated pts or triple-negative ET (20,4% vs 7,2% vs 17,3% respectively, p=0,05). No difference between JAK2, CALR mutated and triple-negative ET was identified in terms of incidence of recurrent thrombosis during follow-up. Compared to those with JAK2 mutation, CALR-mutated ET pts were characterized by younger age (p=0,012), lower leukocyte count (p<0,0001) and hematocrit (p<0,002) and higher platelet count (p<0,0001) and presented a lower IPSET score (p<0,0001). Triple-negative pts showed a low IPSET score, similar to CALR-mutated pts; they had a lower incidence of microvascular symptoms (13.5%) in respect to JAK2 (39,8%) and CALR-mutated (34,8%) pts (p<0,0006 and p=0,014, respectively), with a low intensity of cytoreductive treatment. Interestingly, CALR-mutated ET presented an increased risk of progression to MF (24,6%) in comparison with JAK2V617F positive (5,6%) and triple-negative (7,7%) ET (p<0,0001). CALR type 1 deletion seemed to be strongly associated with MF evolution (15/17 pts). Median MFS seemed shorter in JAK2 positive pts (79,77 months) compared to CALR-mutated (106,44 months) and triple-negative ET pts (96,04 months). As shown in Figure 1, we finally documented a better OS in CALR pts than in JAK2 positive group (median OS 317 vs 251 months respectively; p=0,007). No difference in LFS according to different genotypes was demonstrated.
Conclusion
This study in a large cohort of ET supports and extends the growing body of evidence showing that different mutational genotypes in ET present significant differences with regards to clinical and hematologic presentation as well as risk of MF progression. Even though OS was higher, CALR mutation-positive patients appear to have a higher risk of evolution to MF compared to the JAK2 V617F-positive and triple-negative pts.

Session topic: E-poster
Keyword(s): Essential Thrombocytemia, Myelofibrosis, Myeloproliferative disorder
Abstract: E1340
Type: Eposter Presentation
Background
About 85% of patients (pts) with Essential Thrombocythemia (ET) harbors one of three driver mutations: JAK2, calreticulin (CALR) and MPL; the remaining are defined 'triple-negative'. Recent studies have analyzed the clinical and hematological features of ET or primary myelofibrosis (MF) according to mutational genotype. However the clinical impact of these mutations seems to differ between cases of ET and MF. In particular controversial data were reported regarding the risk of MF progression in triple-negative and CALR mutated ET.
Aims
We retrospectively analyzed a cohort of 350 ET pts, diagnosed between 1990 and 2015 according to WHO classification, and we compared the clinical phenotypes and outcome of CALR mutant cases with the group of JAK2V617F positive and triple-negative pts.
Methods
Hematological parameters, cardiovascular factors, IPSET score, microvascular symptoms and thrombotic complications were reported for each cohorts of ET pts, as well as cytoreductive treatment. During follow-up, progression to MF and leukemic evolution were also recorded. Mann-Whitney test was used for numerical comparisons while Chi-square test was used for nominal ones. Overall survival (OS) and myelofibrosis-free-survival (MFS) and Leukemia-free-survival (LFS) were estimated by Kaplan–Meier analysis and compared with the Log-rank test.
Results
Among 350 ET pts, CALR mutations were detected in 69 (19.7 %), JAK2V617F mutations in 216 (61,7 %) and MPL mutations in 13 (3.7 %). 52 (15%) pts were triple-negative. The median follow-up was 77,5 months, with a longer time of observation for CALR mutants (median 125 months). JAK2-mutated ET had a higher risk of thrombosis at onset, which was about three times higher than CALR mutated pts or triple-negative ET (20,4% vs 7,2% vs 17,3% respectively, p=0,05). No difference between JAK2, CALR mutated and triple-negative ET was identified in terms of incidence of recurrent thrombosis during follow-up. Compared to those with JAK2 mutation, CALR-mutated ET pts were characterized by younger age (p=0,012), lower leukocyte count (p<0,0001) and hematocrit (p<0,002) and higher platelet count (p<0,0001) and presented a lower IPSET score (p<0,0001). Triple-negative pts showed a low IPSET score, similar to CALR-mutated pts; they had a lower incidence of microvascular symptoms (13.5%) in respect to JAK2 (39,8%) and CALR-mutated (34,8%) pts (p<0,0006 and p=0,014, respectively), with a low intensity of cytoreductive treatment. Interestingly, CALR-mutated ET presented an increased risk of progression to MF (24,6%) in comparison with JAK2V617F positive (5,6%) and triple-negative (7,7%) ET (p<0,0001). CALR type 1 deletion seemed to be strongly associated with MF evolution (15/17 pts). Median MFS seemed shorter in JAK2 positive pts (79,77 months) compared to CALR-mutated (106,44 months) and triple-negative ET pts (96,04 months). As shown in Figure 1, we finally documented a better OS in CALR pts than in JAK2 positive group (median OS 317 vs 251 months respectively; p=0,007). No difference in LFS according to different genotypes was demonstrated.
Conclusion
This study in a large cohort of ET supports and extends the growing body of evidence showing that different mutational genotypes in ET present significant differences with regards to clinical and hematologic presentation as well as risk of MF progression. Even though OS was higher, CALR mutation-positive patients appear to have a higher risk of evolution to MF compared to the JAK2 V617F-positive and triple-negative pts.

Session topic: E-poster
Keyword(s): Essential Thrombocytemia, Myelofibrosis, Myeloproliferative disorder
Type: Eposter Presentation
Background
About 85% of patients (pts) with Essential Thrombocythemia (ET) harbors one of three driver mutations: JAK2, calreticulin (CALR) and MPL; the remaining are defined 'triple-negative'. Recent studies have analyzed the clinical and hematological features of ET or primary myelofibrosis (MF) according to mutational genotype. However the clinical impact of these mutations seems to differ between cases of ET and MF. In particular controversial data were reported regarding the risk of MF progression in triple-negative and CALR mutated ET.
Aims
We retrospectively analyzed a cohort of 350 ET pts, diagnosed between 1990 and 2015 according to WHO classification, and we compared the clinical phenotypes and outcome of CALR mutant cases with the group of JAK2V617F positive and triple-negative pts.
Methods
Hematological parameters, cardiovascular factors, IPSET score, microvascular symptoms and thrombotic complications were reported for each cohorts of ET pts, as well as cytoreductive treatment. During follow-up, progression to MF and leukemic evolution were also recorded. Mann-Whitney test was used for numerical comparisons while Chi-square test was used for nominal ones. Overall survival (OS) and myelofibrosis-free-survival (MFS) and Leukemia-free-survival (LFS) were estimated by Kaplan–Meier analysis and compared with the Log-rank test.
Results
Among 350 ET pts, CALR mutations were detected in 69 (19.7 %), JAK2V617F mutations in 216 (61,7 %) and MPL mutations in 13 (3.7 %). 52 (15%) pts were triple-negative. The median follow-up was 77,5 months, with a longer time of observation for CALR mutants (median 125 months). JAK2-mutated ET had a higher risk of thrombosis at onset, which was about three times higher than CALR mutated pts or triple-negative ET (20,4% vs 7,2% vs 17,3% respectively, p=0,05). No difference between JAK2, CALR mutated and triple-negative ET was identified in terms of incidence of recurrent thrombosis during follow-up. Compared to those with JAK2 mutation, CALR-mutated ET pts were characterized by younger age (p=0,012), lower leukocyte count (p<0,0001) and hematocrit (p<0,002) and higher platelet count (p<0,0001) and presented a lower IPSET score (p<0,0001). Triple-negative pts showed a low IPSET score, similar to CALR-mutated pts; they had a lower incidence of microvascular symptoms (13.5%) in respect to JAK2 (39,8%) and CALR-mutated (34,8%) pts (p<0,0006 and p=0,014, respectively), with a low intensity of cytoreductive treatment. Interestingly, CALR-mutated ET presented an increased risk of progression to MF (24,6%) in comparison with JAK2V617F positive (5,6%) and triple-negative (7,7%) ET (p<0,0001). CALR type 1 deletion seemed to be strongly associated with MF evolution (15/17 pts). Median MFS seemed shorter in JAK2 positive pts (79,77 months) compared to CALR-mutated (106,44 months) and triple-negative ET pts (96,04 months). As shown in Figure 1, we finally documented a better OS in CALR pts than in JAK2 positive group (median OS 317 vs 251 months respectively; p=0,007). No difference in LFS according to different genotypes was demonstrated.
Conclusion
This study in a large cohort of ET supports and extends the growing body of evidence showing that different mutational genotypes in ET present significant differences with regards to clinical and hematologic presentation as well as risk of MF progression. Even though OS was higher, CALR mutation-positive patients appear to have a higher risk of evolution to MF compared to the JAK2 V617F-positive and triple-negative pts.

Session topic: E-poster
Keyword(s): Essential Thrombocytemia, Myelofibrosis, Myeloproliferative disorder
{{ help_message }}
{{filter}}


