ABNORMAL EXPRESSION OF LCN2 (NEUTROPHIL GELATINASE-ASSOCIATED LIPOCALIN) IN MYELOPROLIFERATIVE NEOPLASMS
(Abstract release date: 05/19/16)
EHA Library. Fanelli T. 06/09/16; 132881; E1332

Dr. Tiziana Fanelli
Contributions
Contributions
Abstract
Abstract: E1332
Type: Eposter Presentation
Background
Myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), are clonal hematopoietic stem cells’ disorders. Clonal proliferation in MF is accompanied by a secondary inflammatory reaction characterized by bone marrow (BM) stromal changes and abnormal cytokine expression. We recently reported LCN2/NGAL upregulated mRNA expression in CD34+ cells of MF patients (Norfo et al. Blood 2014;124:e21-e32); increased BM expression of LCN2 might facilitate clonal predominance and contribute to a dysfunctional BM microenvironment (Lu M at al. Blood.2015;126:972-982).
Aims
We aimed to investigate the clinical significance of LCN2 levels and its correlation with clinical and molecular aspects in patients with MPNs.
Methods
One hundred well characterized MPN patients (20 PV, 20 ET, 30 PMF, 30 PPV-MF and PET-MF) were included in this study, and 20 health subjects. LCN2 mRNA expression was performed in granulocytes by RTQ-PCR. Measurements of NGAL plasma protein (PP) were performed by ELISA (Bioporto Diagnostics). Mutational status of JAK2, MPL, EZH2, ASXL1, IDH1/2, CBL, TP53, TET2, DNMT3A, SRSF2 was evaluated as previously reported. Statistical analyses were conducted with SPSS and Origin software.
Results
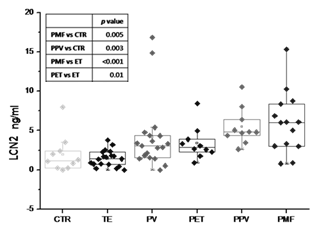
Significantly higher levels of LCN2 mRNA and PP were found in PMF pts (mRNA: median RQ 9.70; PP: median 6.03ng/ml) in comparison with controls (mRNA:median RQ 1.09, P=.02; PP: median 1.19ng/ml; P=.005). mRNA LCN2 expression in PMF was also significantly higher compared with PV and ET (median RQ 1.89; P=.01 and median RQ 3.55; P=.02, respectively). Higher levels of PP were also observed in PPV-MF (median 4.82ng/ml) compared to controls (P=.003). LCN2 PP levels comparison among MPN sub-types is reported in Figure1. In particular LCN2 PP levels in PET-MF (median 2.86 ng/ml) were two-fold greater than in ET (median 1.395 ng/ml; P= .01), suggesting a link between increased LCN2 levels and progression to MF. mRNA expression inversely correlated with hemoglobin levels (P=.03, r=-0.29). LCN2 mRNA levels were positively correlated with IPSS scores (P=.01), and were higher in ASXL1+ pts (n=23) compared with WT (3.2 vs 10.4, P=.02). CALR+ pts showed lower levels compared with JAK2+ or Triple Negative (2.60 vs 4.85 vs 8.71 ng/ml respectively, P=.04), while PP levels were significantly higher in SRSF2+ pts (n=4) compared to WT (17.35 vs 4.04, P=.02). PP levels were linearly correlated with CD34+ count (P=.01, r=0.28). In 17 of 33 MF pts, reduction of PP LCN2 levels was observed after treatment with ruxolitinib; of these 6 (54.5%) achieved a spleen volume reduction and in 7 (63.6%) constitutional symptoms resolved.
Conclusion
Lipocalin might represent a novel diagnostic and/or prognostic marker and could also provide a new therapeutic target in MPNs, particularly myelofibrosis. Further studies are required to fully clarify the clinical significance of Lipocalin overexpression in myelofibrosis.
Session topic: E-poster
Keyword(s): Myelofibrosis, Myeloproliferative disorder, Prognostic factor
Type: Eposter Presentation
Background
Myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), are clonal hematopoietic stem cells’ disorders. Clonal proliferation in MF is accompanied by a secondary inflammatory reaction characterized by bone marrow (BM) stromal changes and abnormal cytokine expression. We recently reported LCN2/NGAL upregulated mRNA expression in CD34+ cells of MF patients (Norfo et al. Blood 2014;124:e21-e32); increased BM expression of LCN2 might facilitate clonal predominance and contribute to a dysfunctional BM microenvironment (Lu M at al. Blood.2015;126:972-982).
Aims
We aimed to investigate the clinical significance of LCN2 levels and its correlation with clinical and molecular aspects in patients with MPNs.
Methods
One hundred well characterized MPN patients (20 PV, 20 ET, 30 PMF, 30 PPV-MF and PET-MF) were included in this study, and 20 health subjects. LCN2 mRNA expression was performed in granulocytes by RTQ-PCR. Measurements of NGAL plasma protein (PP) were performed by ELISA (Bioporto Diagnostics). Mutational status of JAK2, MPL, EZH2, ASXL1, IDH1/2, CBL, TP53, TET2, DNMT3A, SRSF2 was evaluated as previously reported. Statistical analyses were conducted with SPSS and Origin software.
Results
Significantly higher levels of LCN2 mRNA and PP were found in PMF pts (mRNA: median RQ 9.70; PP: median 6.03ng/ml) in comparison with controls (mRNA:median RQ 1.09, P=.02; PP: median 1.19ng/ml; P=.005). mRNA LCN2 expression in PMF was also significantly higher compared with PV and ET (median RQ 1.89; P=.01 and median RQ 3.55; P=.02, respectively). Higher levels of PP were also observed in PPV-MF (median 4.82ng/ml) compared to controls (P=.003). LCN2 PP levels comparison among MPN sub-types is reported in Figure1. In particular LCN2 PP levels in PET-MF (median 2.86 ng/ml) were two-fold greater than in ET (median 1.395 ng/ml; P= .01), suggesting a link between increased LCN2 levels and progression to MF. mRNA expression inversely correlated with hemoglobin levels (P=.03, r=-0.29). LCN2 mRNA levels were positively correlated with IPSS scores (P=.01), and were higher in ASXL1+ pts (n=23) compared with WT (3.2 vs 10.4, P=.02). CALR+ pts showed lower levels compared with JAK2+ or Triple Negative (2.60 vs 4.85 vs 8.71 ng/ml respectively, P=.04), while PP levels were significantly higher in SRSF2+ pts (n=4) compared to WT (17.35 vs 4.04, P=.02). PP levels were linearly correlated with CD34+ count (P=.01, r=0.28). In 17 of 33 MF pts, reduction of PP LCN2 levels was observed after treatment with ruxolitinib; of these 6 (54.5%) achieved a spleen volume reduction and in 7 (63.6%) constitutional symptoms resolved.
Conclusion
Lipocalin might represent a novel diagnostic and/or prognostic marker and could also provide a new therapeutic target in MPNs, particularly myelofibrosis. Further studies are required to fully clarify the clinical significance of Lipocalin overexpression in myelofibrosis.

Session topic: E-poster
Keyword(s): Myelofibrosis, Myeloproliferative disorder, Prognostic factor
Abstract: E1332
Type: Eposter Presentation
Background
Myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), are clonal hematopoietic stem cells’ disorders. Clonal proliferation in MF is accompanied by a secondary inflammatory reaction characterized by bone marrow (BM) stromal changes and abnormal cytokine expression. We recently reported LCN2/NGAL upregulated mRNA expression in CD34+ cells of MF patients (Norfo et al. Blood 2014;124:e21-e32); increased BM expression of LCN2 might facilitate clonal predominance and contribute to a dysfunctional BM microenvironment (Lu M at al. Blood.2015;126:972-982).
Aims
We aimed to investigate the clinical significance of LCN2 levels and its correlation with clinical and molecular aspects in patients with MPNs.
Methods
One hundred well characterized MPN patients (20 PV, 20 ET, 30 PMF, 30 PPV-MF and PET-MF) were included in this study, and 20 health subjects. LCN2 mRNA expression was performed in granulocytes by RTQ-PCR. Measurements of NGAL plasma protein (PP) were performed by ELISA (Bioporto Diagnostics). Mutational status of JAK2, MPL, EZH2, ASXL1, IDH1/2, CBL, TP53, TET2, DNMT3A, SRSF2 was evaluated as previously reported. Statistical analyses were conducted with SPSS and Origin software.
Results
Significantly higher levels of LCN2 mRNA and PP were found in PMF pts (mRNA: median RQ 9.70; PP: median 6.03ng/ml) in comparison with controls (mRNA:median RQ 1.09, P=.02; PP: median 1.19ng/ml; P=.005). mRNA LCN2 expression in PMF was also significantly higher compared with PV and ET (median RQ 1.89; P=.01 and median RQ 3.55; P=.02, respectively). Higher levels of PP were also observed in PPV-MF (median 4.82ng/ml) compared to controls (P=.003). LCN2 PP levels comparison among MPN sub-types is reported in Figure1. In particular LCN2 PP levels in PET-MF (median 2.86 ng/ml) were two-fold greater than in ET (median 1.395 ng/ml; P= .01), suggesting a link between increased LCN2 levels and progression to MF. mRNA expression inversely correlated with hemoglobin levels (P=.03, r=-0.29). LCN2 mRNA levels were positively correlated with IPSS scores (P=.01), and were higher in ASXL1+ pts (n=23) compared with WT (3.2 vs 10.4, P=.02). CALR+ pts showed lower levels compared with JAK2+ or Triple Negative (2.60 vs 4.85 vs 8.71 ng/ml respectively, P=.04), while PP levels were significantly higher in SRSF2+ pts (n=4) compared to WT (17.35 vs 4.04, P=.02). PP levels were linearly correlated with CD34+ count (P=.01, r=0.28). In 17 of 33 MF pts, reduction of PP LCN2 levels was observed after treatment with ruxolitinib; of these 6 (54.5%) achieved a spleen volume reduction and in 7 (63.6%) constitutional symptoms resolved.
Conclusion
Lipocalin might represent a novel diagnostic and/or prognostic marker and could also provide a new therapeutic target in MPNs, particularly myelofibrosis. Further studies are required to fully clarify the clinical significance of Lipocalin overexpression in myelofibrosis.

Session topic: E-poster
Keyword(s): Myelofibrosis, Myeloproliferative disorder, Prognostic factor
Type: Eposter Presentation
Background
Myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), are clonal hematopoietic stem cells’ disorders. Clonal proliferation in MF is accompanied by a secondary inflammatory reaction characterized by bone marrow (BM) stromal changes and abnormal cytokine expression. We recently reported LCN2/NGAL upregulated mRNA expression in CD34+ cells of MF patients (Norfo et al. Blood 2014;124:e21-e32); increased BM expression of LCN2 might facilitate clonal predominance and contribute to a dysfunctional BM microenvironment (Lu M at al. Blood.2015;126:972-982).
Aims
We aimed to investigate the clinical significance of LCN2 levels and its correlation with clinical and molecular aspects in patients with MPNs.
Methods
One hundred well characterized MPN patients (20 PV, 20 ET, 30 PMF, 30 PPV-MF and PET-MF) were included in this study, and 20 health subjects. LCN2 mRNA expression was performed in granulocytes by RTQ-PCR. Measurements of NGAL plasma protein (PP) were performed by ELISA (Bioporto Diagnostics). Mutational status of JAK2, MPL, EZH2, ASXL1, IDH1/2, CBL, TP53, TET2, DNMT3A, SRSF2 was evaluated as previously reported. Statistical analyses were conducted with SPSS and Origin software.
Results
Significantly higher levels of LCN2 mRNA and PP were found in PMF pts (mRNA: median RQ 9.70; PP: median 6.03ng/ml) in comparison with controls (mRNA:median RQ 1.09, P=.02; PP: median 1.19ng/ml; P=.005). mRNA LCN2 expression in PMF was also significantly higher compared with PV and ET (median RQ 1.89; P=.01 and median RQ 3.55; P=.02, respectively). Higher levels of PP were also observed in PPV-MF (median 4.82ng/ml) compared to controls (P=.003). LCN2 PP levels comparison among MPN sub-types is reported in Figure1. In particular LCN2 PP levels in PET-MF (median 2.86 ng/ml) were two-fold greater than in ET (median 1.395 ng/ml; P= .01), suggesting a link between increased LCN2 levels and progression to MF. mRNA expression inversely correlated with hemoglobin levels (P=.03, r=-0.29). LCN2 mRNA levels were positively correlated with IPSS scores (P=.01), and were higher in ASXL1+ pts (n=23) compared with WT (3.2 vs 10.4, P=.02). CALR+ pts showed lower levels compared with JAK2+ or Triple Negative (2.60 vs 4.85 vs 8.71 ng/ml respectively, P=.04), while PP levels were significantly higher in SRSF2+ pts (n=4) compared to WT (17.35 vs 4.04, P=.02). PP levels were linearly correlated with CD34+ count (P=.01, r=0.28). In 17 of 33 MF pts, reduction of PP LCN2 levels was observed after treatment with ruxolitinib; of these 6 (54.5%) achieved a spleen volume reduction and in 7 (63.6%) constitutional symptoms resolved.
Conclusion
Lipocalin might represent a novel diagnostic and/or prognostic marker and could also provide a new therapeutic target in MPNs, particularly myelofibrosis. Further studies are required to fully clarify the clinical significance of Lipocalin overexpression in myelofibrosis.

Session topic: E-poster
Keyword(s): Myelofibrosis, Myeloproliferative disorder, Prognostic factor
{{ help_message }}
{{filter}}


