BETTER OUTCOME IN AL AMYLOIDOSIS (ALA) TREATED WITH MULTIPLE MYELOMA-ORIENTED REGIMENS COMPARED WITH CYCLOPHOSPHAMIDE/BORTEZOMIB/DEXAMETHASONE (CYBORD) IN A SINGLE CENTER EXPERIENCE
(Abstract release date: 05/19/16)
EHA Library. BELOTTI A. 06/09/16; 132866; E1317

Dr. ANGELO BELOTTI
Contributions
Contributions
Abstract
Abstract: E1317
Type: Eposter Presentation
Background
Treatment of AL amyloidosis (ALA) is based on anti-myeloma therapy but there is no standard. Treatment-related toxicity is deemed to be higher in pts with ALA compared with patients with multiple myeloma (MM). Recently, the combination of cyclophosphamide-bortezomib-dexamethasone (CyBorD) showed high rates of haematologic response, although the outcome of high risk patients remained poor.
Aims
we compared the outcome of patients treated at our center with CyBorD with patients receiving more intensive therapy, including MM-type chemotherapy regimens followed or not by high doses of melphalan (HD-Mel) and autologous stem cell transplantation (ASCT) or upfront HD-Mel with ASCT.
Methods
Of 50 ALA patients (median age 62 years) referred to our center, 48 of whom newly diagnosed between 2007 and 2015, 25 patients received CyBorD, and 5 of them (20%) subsequently underwent ASCT. By intention to treat, 18 patients (control group) received more intensive treatment (upfront ASCT: 6 (33%); conventional myeloma induction therapy (VAD, VTD, VMP): 12 (67%), followed by ASCT in 5 of them, 42%). Seven patients receiving less intensive therapy were excluded from analysis. Risk groups were identified as follows: cTnI >0.1ng/ml and/or ECOG PS ≥3: high risk; age ≤65 years with normal cTnI levels, ECOG PS <3 and eGFR > 50ml/min: low risk. Intermediate risk patients were defined if not meeting criteria for high or low risk. MM diagnosis was made according to IMWG criteria. Haematological and organ response, overall survival (OS) and event free survival (EFS: time to 2nd line therapy or death) were analyzed.
Results
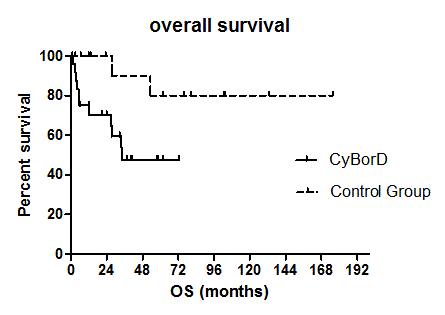
The proportion of high risk patients was 32% in the CyBorD and 28% in the control group. Median age was 62 and 61 years in the CyBorD group and in the control group, respectively. Concomitant MM was present in 52% CyBorD and in 94% control patients. Treatment results of CyBorD patients were in line with those recently reported (Palladini et al, Blood 2015). Compared to the control group, no difference was observed in terms of haematologic response. Overall response rate (ORR) and complete remission (CR) were 72% and 20% in the CyBorD group and 89% and 39% in the control group, respectively. However, organ response rate was significantly lower in the CyBorD group (32% vs 75%, p 0.01). Toxicity was manageable in both groups. After a median follow up of 33 months no significant difference was seen between CyBorD and control group in terms of EFS (48% vs 61% at 2 years, respectively; HR 1.5, 95% CI 0.66-3.41), whereas OS was significantly inferior in the CyBorD group (47.6% vs 80% at 4 years, p 0.027, HR 3.45, 95% CI 1.15-10.36, see Figure 1). Excluding high risk patients in both groups, OS resulted similar, although a trend toward better long term survival was seen in the control group.Figure 1- Overall Survival according to treatment: CyBorD vs MM-type chemotherapy (control group)
Conclusion
despite the high haematologic response rates, CyBorD regimen seems not to overcome the long term poor prognosis associated with organ damage in ALA. of high risk patients. Selected patients, even in the high-risk group may have a survival advantage with MM conventional treatment, including ASCT whenever possible.
Session topic: E-poster
Keyword(s): AL amyloidosis, Amyloidosis, Myeloma
Type: Eposter Presentation
Background
Treatment of AL amyloidosis (ALA) is based on anti-myeloma therapy but there is no standard. Treatment-related toxicity is deemed to be higher in pts with ALA compared with patients with multiple myeloma (MM). Recently, the combination of cyclophosphamide-bortezomib-dexamethasone (CyBorD) showed high rates of haematologic response, although the outcome of high risk patients remained poor.
Aims
we compared the outcome of patients treated at our center with CyBorD with patients receiving more intensive therapy, including MM-type chemotherapy regimens followed or not by high doses of melphalan (HD-Mel) and autologous stem cell transplantation (ASCT) or upfront HD-Mel with ASCT.
Methods
Of 50 ALA patients (median age 62 years) referred to our center, 48 of whom newly diagnosed between 2007 and 2015, 25 patients received CyBorD, and 5 of them (20%) subsequently underwent ASCT. By intention to treat, 18 patients (control group) received more intensive treatment (upfront ASCT: 6 (33%); conventional myeloma induction therapy (VAD, VTD, VMP): 12 (67%), followed by ASCT in 5 of them, 42%). Seven patients receiving less intensive therapy were excluded from analysis. Risk groups were identified as follows: cTnI >0.1ng/ml and/or ECOG PS ≥3: high risk; age ≤65 years with normal cTnI levels, ECOG PS <3 and eGFR > 50ml/min: low risk. Intermediate risk patients were defined if not meeting criteria for high or low risk. MM diagnosis was made according to IMWG criteria. Haematological and organ response, overall survival (OS) and event free survival (EFS: time to 2nd line therapy or death) were analyzed.
Results
The proportion of high risk patients was 32% in the CyBorD and 28% in the control group. Median age was 62 and 61 years in the CyBorD group and in the control group, respectively. Concomitant MM was present in 52% CyBorD and in 94% control patients. Treatment results of CyBorD patients were in line with those recently reported (Palladini et al, Blood 2015). Compared to the control group, no difference was observed in terms of haematologic response. Overall response rate (ORR) and complete remission (CR) were 72% and 20% in the CyBorD group and 89% and 39% in the control group, respectively. However, organ response rate was significantly lower in the CyBorD group (32% vs 75%, p 0.01). Toxicity was manageable in both groups. After a median follow up of 33 months no significant difference was seen between CyBorD and control group in terms of EFS (48% vs 61% at 2 years, respectively; HR 1.5, 95% CI 0.66-3.41), whereas OS was significantly inferior in the CyBorD group (47.6% vs 80% at 4 years, p 0.027, HR 3.45, 95% CI 1.15-10.36, see Figure 1). Excluding high risk patients in both groups, OS resulted similar, although a trend toward better long term survival was seen in the control group.Figure 1- Overall Survival according to treatment: CyBorD vs MM-type chemotherapy (control group)
Conclusion
despite the high haematologic response rates, CyBorD regimen seems not to overcome the long term poor prognosis associated with organ damage in ALA. of high risk patients. Selected patients, even in the high-risk group may have a survival advantage with MM conventional treatment, including ASCT whenever possible.

Session topic: E-poster
Keyword(s): AL amyloidosis, Amyloidosis, Myeloma
Abstract: E1317
Type: Eposter Presentation
Background
Treatment of AL amyloidosis (ALA) is based on anti-myeloma therapy but there is no standard. Treatment-related toxicity is deemed to be higher in pts with ALA compared with patients with multiple myeloma (MM). Recently, the combination of cyclophosphamide-bortezomib-dexamethasone (CyBorD) showed high rates of haematologic response, although the outcome of high risk patients remained poor.
Aims
we compared the outcome of patients treated at our center with CyBorD with patients receiving more intensive therapy, including MM-type chemotherapy regimens followed or not by high doses of melphalan (HD-Mel) and autologous stem cell transplantation (ASCT) or upfront HD-Mel with ASCT.
Methods
Of 50 ALA patients (median age 62 years) referred to our center, 48 of whom newly diagnosed between 2007 and 2015, 25 patients received CyBorD, and 5 of them (20%) subsequently underwent ASCT. By intention to treat, 18 patients (control group) received more intensive treatment (upfront ASCT: 6 (33%); conventional myeloma induction therapy (VAD, VTD, VMP): 12 (67%), followed by ASCT in 5 of them, 42%). Seven patients receiving less intensive therapy were excluded from analysis. Risk groups were identified as follows: cTnI >0.1ng/ml and/or ECOG PS ≥3: high risk; age ≤65 years with normal cTnI levels, ECOG PS <3 and eGFR > 50ml/min: low risk. Intermediate risk patients were defined if not meeting criteria for high or low risk. MM diagnosis was made according to IMWG criteria. Haematological and organ response, overall survival (OS) and event free survival (EFS: time to 2nd line therapy or death) were analyzed.
Results
The proportion of high risk patients was 32% in the CyBorD and 28% in the control group. Median age was 62 and 61 years in the CyBorD group and in the control group, respectively. Concomitant MM was present in 52% CyBorD and in 94% control patients. Treatment results of CyBorD patients were in line with those recently reported (Palladini et al, Blood 2015). Compared to the control group, no difference was observed in terms of haematologic response. Overall response rate (ORR) and complete remission (CR) were 72% and 20% in the CyBorD group and 89% and 39% in the control group, respectively. However, organ response rate was significantly lower in the CyBorD group (32% vs 75%, p 0.01). Toxicity was manageable in both groups. After a median follow up of 33 months no significant difference was seen between CyBorD and control group in terms of EFS (48% vs 61% at 2 years, respectively; HR 1.5, 95% CI 0.66-3.41), whereas OS was significantly inferior in the CyBorD group (47.6% vs 80% at 4 years, p 0.027, HR 3.45, 95% CI 1.15-10.36, see Figure 1). Excluding high risk patients in both groups, OS resulted similar, although a trend toward better long term survival was seen in the control group.Figure 1- Overall Survival according to treatment: CyBorD vs MM-type chemotherapy (control group)
Conclusion
despite the high haematologic response rates, CyBorD regimen seems not to overcome the long term poor prognosis associated with organ damage in ALA. of high risk patients. Selected patients, even in the high-risk group may have a survival advantage with MM conventional treatment, including ASCT whenever possible.

Session topic: E-poster
Keyword(s): AL amyloidosis, Amyloidosis, Myeloma
Type: Eposter Presentation
Background
Treatment of AL amyloidosis (ALA) is based on anti-myeloma therapy but there is no standard. Treatment-related toxicity is deemed to be higher in pts with ALA compared with patients with multiple myeloma (MM). Recently, the combination of cyclophosphamide-bortezomib-dexamethasone (CyBorD) showed high rates of haematologic response, although the outcome of high risk patients remained poor.
Aims
we compared the outcome of patients treated at our center with CyBorD with patients receiving more intensive therapy, including MM-type chemotherapy regimens followed or not by high doses of melphalan (HD-Mel) and autologous stem cell transplantation (ASCT) or upfront HD-Mel with ASCT.
Methods
Of 50 ALA patients (median age 62 years) referred to our center, 48 of whom newly diagnosed between 2007 and 2015, 25 patients received CyBorD, and 5 of them (20%) subsequently underwent ASCT. By intention to treat, 18 patients (control group) received more intensive treatment (upfront ASCT: 6 (33%); conventional myeloma induction therapy (VAD, VTD, VMP): 12 (67%), followed by ASCT in 5 of them, 42%). Seven patients receiving less intensive therapy were excluded from analysis. Risk groups were identified as follows: cTnI >0.1ng/ml and/or ECOG PS ≥3: high risk; age ≤65 years with normal cTnI levels, ECOG PS <3 and eGFR > 50ml/min: low risk. Intermediate risk patients were defined if not meeting criteria for high or low risk. MM diagnosis was made according to IMWG criteria. Haematological and organ response, overall survival (OS) and event free survival (EFS: time to 2nd line therapy or death) were analyzed.
Results
The proportion of high risk patients was 32% in the CyBorD and 28% in the control group. Median age was 62 and 61 years in the CyBorD group and in the control group, respectively. Concomitant MM was present in 52% CyBorD and in 94% control patients. Treatment results of CyBorD patients were in line with those recently reported (Palladini et al, Blood 2015). Compared to the control group, no difference was observed in terms of haematologic response. Overall response rate (ORR) and complete remission (CR) were 72% and 20% in the CyBorD group and 89% and 39% in the control group, respectively. However, organ response rate was significantly lower in the CyBorD group (32% vs 75%, p 0.01). Toxicity was manageable in both groups. After a median follow up of 33 months no significant difference was seen between CyBorD and control group in terms of EFS (48% vs 61% at 2 years, respectively; HR 1.5, 95% CI 0.66-3.41), whereas OS was significantly inferior in the CyBorD group (47.6% vs 80% at 4 years, p 0.027, HR 3.45, 95% CI 1.15-10.36, see Figure 1). Excluding high risk patients in both groups, OS resulted similar, although a trend toward better long term survival was seen in the control group.Figure 1- Overall Survival according to treatment: CyBorD vs MM-type chemotherapy (control group)
Conclusion
despite the high haematologic response rates, CyBorD regimen seems not to overcome the long term poor prognosis associated with organ damage in ALA. of high risk patients. Selected patients, even in the high-risk group may have a survival advantage with MM conventional treatment, including ASCT whenever possible.

Session topic: E-poster
Keyword(s): AL amyloidosis, Amyloidosis, Myeloma
{{ help_message }}
{{filter}}


