PROSPECTIVE FUNCTIONAL GERIATRIC ASSESSMENT (CF-GA) IN MULTIPLE MYELOMA (MM) PATIENTS (PTS): CHANGES FROM BASELINE (T0) TO FOLLOW UP ASSESSMENT (T1)
(Abstract release date: 05/19/16)
EHA Library. Dold S. 06/09/16; 132845; E1296

Ms. Sandra Dold
Contributions
Contributions
Abstract
Abstract: E1296
Type: Eposter Presentation
Background
Multiple myeloma (MM) is a hematologic malignancy, its incidence increasing with age. MM patients (pts) present with heterogeneous health status, whereby defined tests help to objectively define biological fitness, rather than considering pts' chronological age. This is specifically relevant, since treatment options are numerous today. For the discrimination of current therapies, a variety of MM- and patient-related risk factors, such as comorbidities (CM) and frailty can be considered. Therefore, the careful assessment of individual conditions before treatment and with subsequent, defined follow-up seems relevant. Therapy-related complications and early mortality may substantially increase in pts with CM, but may also change during treatment.
Aims
Our aim is to establish a prospective comorbidity and functional geriatric assesment (CF-GA) for MM pts and to show whether the GA shows substantial changes from baseline (T0) to follow-up (T1 and subsequently T2) and which tests are most indicative. This has - to the best of our knowledge - never been assessed in MM.
Methods
This prospective intervention trial performed a simple, defined GA in consecutive MM pts prior to initiation of antimyeloma treatment and during subsequent follow-up (6-12 [T1] and 24 months [T2]). The GA included the Karnofsky Performance Status (KPS), pain scale, rating of fitness, IADL, ADL, malnutrition, geriatric depression scale (GDS), mini-mental status (MMS) and established comorbidity (CM) scores: Charlson Comorbidity Index (CCI), Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), Myeloma Comorbidity Index (initial-MCI [I-MCI] and revised-MCI [R-MCI]; http://www.myelomacomorbidityindex.org) and Kaplan Feinstein (KF). The trial was approved by the ethics committees of the Freiburg University. All patients provided written informed consent and all procedures were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and Guidelines for Good Clinical Practice.
Results
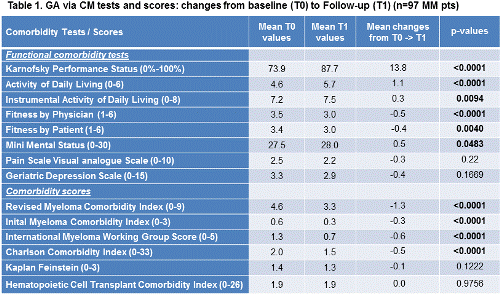
Currently a total of 97 consecutive pts have been included in this GA, all receiving both T0- and T1-assessment (median 12 months after treatment initiation). The median pt age was 60.3 years (27-83). Between T0 and T1, 54 pts had received stem cell transplants (n=49: ASCT, n=1: allo-SCT, n=4 auto-allo-SCT); and the other half of pts novel agent-containing, standard chemotherapy and/or regular surveillance. Significant changes between T0- to T1-assessments were observed for various GA tests as summarized in Table 1. Of note, all mean test and score results improved, most notably the I-MCI, R-MCI-, IMWG- and CCI-scores, whereas the Kaplan Feinstein and HCT-CI remained almost those of baseline levels. Moreover, the KPS, ADL, IADL, fitness as rated both by physicians and patients as well as MMS improved. Of interest, the fitness rating by physicians seemed to improve more substantially than that given by pts, demonstrating again that a brief GA assessment with simple tests is more objective than clinical judgement alone. Pain, depression and malnutrition also improved, albeit to lesser and non-significant extends.
Conclusion
Our CF-GA contains simple, reliable tests, which consistently and objectively test MM pts' fitness before and after treatment. Our results impressively demonstrate that MM pts' general and specific fitness - as measured via defined GA tests - may improve during follow-up. This was induced by the implemented antimyeloma treatment, best supportive care measures and lower disease-specific symptoms after therapy. We continue this GA in an even larger prospective multicenter cohort to determine each test's predictive power for survival and treatment toxicity.
Session topic: E-poster
Keyword(s): Comorbidities, Elderly, Multiple myeloma
Type: Eposter Presentation
Background
Multiple myeloma (MM) is a hematologic malignancy, its incidence increasing with age. MM patients (pts) present with heterogeneous health status, whereby defined tests help to objectively define biological fitness, rather than considering pts' chronological age. This is specifically relevant, since treatment options are numerous today. For the discrimination of current therapies, a variety of MM- and patient-related risk factors, such as comorbidities (CM) and frailty can be considered. Therefore, the careful assessment of individual conditions before treatment and with subsequent, defined follow-up seems relevant. Therapy-related complications and early mortality may substantially increase in pts with CM, but may also change during treatment.
Aims
Our aim is to establish a prospective comorbidity and functional geriatric assesment (CF-GA) for MM pts and to show whether the GA shows substantial changes from baseline (T0) to follow-up (T1 and subsequently T2) and which tests are most indicative. This has - to the best of our knowledge - never been assessed in MM.
Methods
This prospective intervention trial performed a simple, defined GA in consecutive MM pts prior to initiation of antimyeloma treatment and during subsequent follow-up (6-12 [T1] and 24 months [T2]). The GA included the Karnofsky Performance Status (KPS), pain scale, rating of fitness, IADL, ADL, malnutrition, geriatric depression scale (GDS), mini-mental status (MMS) and established comorbidity (CM) scores: Charlson Comorbidity Index (CCI), Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), Myeloma Comorbidity Index (initial-MCI [I-MCI] and revised-MCI [R-MCI]; http://www.myelomacomorbidityindex.org) and Kaplan Feinstein (KF). The trial was approved by the ethics committees of the Freiburg University. All patients provided written informed consent and all procedures were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and Guidelines for Good Clinical Practice.
Results
Currently a total of 97 consecutive pts have been included in this GA, all receiving both T0- and T1-assessment (median 12 months after treatment initiation). The median pt age was 60.3 years (27-83). Between T0 and T1, 54 pts had received stem cell transplants (n=49: ASCT, n=1: allo-SCT, n=4 auto-allo-SCT); and the other half of pts novel agent-containing, standard chemotherapy and/or regular surveillance. Significant changes between T0- to T1-assessments were observed for various GA tests as summarized in Table 1. Of note, all mean test and score results improved, most notably the I-MCI, R-MCI-, IMWG- and CCI-scores, whereas the Kaplan Feinstein and HCT-CI remained almost those of baseline levels. Moreover, the KPS, ADL, IADL, fitness as rated both by physicians and patients as well as MMS improved. Of interest, the fitness rating by physicians seemed to improve more substantially than that given by pts, demonstrating again that a brief GA assessment with simple tests is more objective than clinical judgement alone. Pain, depression and malnutrition also improved, albeit to lesser and non-significant extends.
Conclusion
Our CF-GA contains simple, reliable tests, which consistently and objectively test MM pts' fitness before and after treatment. Our results impressively demonstrate that MM pts' general and specific fitness - as measured via defined GA tests - may improve during follow-up. This was induced by the implemented antimyeloma treatment, best supportive care measures and lower disease-specific symptoms after therapy. We continue this GA in an even larger prospective multicenter cohort to determine each test's predictive power for survival and treatment toxicity.

Session topic: E-poster
Keyword(s): Comorbidities, Elderly, Multiple myeloma
Abstract: E1296
Type: Eposter Presentation
Background
Multiple myeloma (MM) is a hematologic malignancy, its incidence increasing with age. MM patients (pts) present with heterogeneous health status, whereby defined tests help to objectively define biological fitness, rather than considering pts' chronological age. This is specifically relevant, since treatment options are numerous today. For the discrimination of current therapies, a variety of MM- and patient-related risk factors, such as comorbidities (CM) and frailty can be considered. Therefore, the careful assessment of individual conditions before treatment and with subsequent, defined follow-up seems relevant. Therapy-related complications and early mortality may substantially increase in pts with CM, but may also change during treatment.
Aims
Our aim is to establish a prospective comorbidity and functional geriatric assesment (CF-GA) for MM pts and to show whether the GA shows substantial changes from baseline (T0) to follow-up (T1 and subsequently T2) and which tests are most indicative. This has - to the best of our knowledge - never been assessed in MM.
Methods
This prospective intervention trial performed a simple, defined GA in consecutive MM pts prior to initiation of antimyeloma treatment and during subsequent follow-up (6-12 [T1] and 24 months [T2]). The GA included the Karnofsky Performance Status (KPS), pain scale, rating of fitness, IADL, ADL, malnutrition, geriatric depression scale (GDS), mini-mental status (MMS) and established comorbidity (CM) scores: Charlson Comorbidity Index (CCI), Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), Myeloma Comorbidity Index (initial-MCI [I-MCI] and revised-MCI [R-MCI]; http://www.myelomacomorbidityindex.org) and Kaplan Feinstein (KF). The trial was approved by the ethics committees of the Freiburg University. All patients provided written informed consent and all procedures were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and Guidelines for Good Clinical Practice.
Results
Currently a total of 97 consecutive pts have been included in this GA, all receiving both T0- and T1-assessment (median 12 months after treatment initiation). The median pt age was 60.3 years (27-83). Between T0 and T1, 54 pts had received stem cell transplants (n=49: ASCT, n=1: allo-SCT, n=4 auto-allo-SCT); and the other half of pts novel agent-containing, standard chemotherapy and/or regular surveillance. Significant changes between T0- to T1-assessments were observed for various GA tests as summarized in Table 1. Of note, all mean test and score results improved, most notably the I-MCI, R-MCI-, IMWG- and CCI-scores, whereas the Kaplan Feinstein and HCT-CI remained almost those of baseline levels. Moreover, the KPS, ADL, IADL, fitness as rated both by physicians and patients as well as MMS improved. Of interest, the fitness rating by physicians seemed to improve more substantially than that given by pts, demonstrating again that a brief GA assessment with simple tests is more objective than clinical judgement alone. Pain, depression and malnutrition also improved, albeit to lesser and non-significant extends.
Conclusion
Our CF-GA contains simple, reliable tests, which consistently and objectively test MM pts' fitness before and after treatment. Our results impressively demonstrate that MM pts' general and specific fitness - as measured via defined GA tests - may improve during follow-up. This was induced by the implemented antimyeloma treatment, best supportive care measures and lower disease-specific symptoms after therapy. We continue this GA in an even larger prospective multicenter cohort to determine each test's predictive power for survival and treatment toxicity.

Session topic: E-poster
Keyword(s): Comorbidities, Elderly, Multiple myeloma
Type: Eposter Presentation
Background
Multiple myeloma (MM) is a hematologic malignancy, its incidence increasing with age. MM patients (pts) present with heterogeneous health status, whereby defined tests help to objectively define biological fitness, rather than considering pts' chronological age. This is specifically relevant, since treatment options are numerous today. For the discrimination of current therapies, a variety of MM- and patient-related risk factors, such as comorbidities (CM) and frailty can be considered. Therefore, the careful assessment of individual conditions before treatment and with subsequent, defined follow-up seems relevant. Therapy-related complications and early mortality may substantially increase in pts with CM, but may also change during treatment.
Aims
Our aim is to establish a prospective comorbidity and functional geriatric assesment (CF-GA) for MM pts and to show whether the GA shows substantial changes from baseline (T0) to follow-up (T1 and subsequently T2) and which tests are most indicative. This has - to the best of our knowledge - never been assessed in MM.
Methods
This prospective intervention trial performed a simple, defined GA in consecutive MM pts prior to initiation of antimyeloma treatment and during subsequent follow-up (6-12 [T1] and 24 months [T2]). The GA included the Karnofsky Performance Status (KPS), pain scale, rating of fitness, IADL, ADL, malnutrition, geriatric depression scale (GDS), mini-mental status (MMS) and established comorbidity (CM) scores: Charlson Comorbidity Index (CCI), Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), Myeloma Comorbidity Index (initial-MCI [I-MCI] and revised-MCI [R-MCI]; http://www.myelomacomorbidityindex.org) and Kaplan Feinstein (KF). The trial was approved by the ethics committees of the Freiburg University. All patients provided written informed consent and all procedures were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and Guidelines for Good Clinical Practice.
Results
Currently a total of 97 consecutive pts have been included in this GA, all receiving both T0- and T1-assessment (median 12 months after treatment initiation). The median pt age was 60.3 years (27-83). Between T0 and T1, 54 pts had received stem cell transplants (n=49: ASCT, n=1: allo-SCT, n=4 auto-allo-SCT); and the other half of pts novel agent-containing, standard chemotherapy and/or regular surveillance. Significant changes between T0- to T1-assessments were observed for various GA tests as summarized in Table 1. Of note, all mean test and score results improved, most notably the I-MCI, R-MCI-, IMWG- and CCI-scores, whereas the Kaplan Feinstein and HCT-CI remained almost those of baseline levels. Moreover, the KPS, ADL, IADL, fitness as rated both by physicians and patients as well as MMS improved. Of interest, the fitness rating by physicians seemed to improve more substantially than that given by pts, demonstrating again that a brief GA assessment with simple tests is more objective than clinical judgement alone. Pain, depression and malnutrition also improved, albeit to lesser and non-significant extends.
Conclusion
Our CF-GA contains simple, reliable tests, which consistently and objectively test MM pts' fitness before and after treatment. Our results impressively demonstrate that MM pts' general and specific fitness - as measured via defined GA tests - may improve during follow-up. This was induced by the implemented antimyeloma treatment, best supportive care measures and lower disease-specific symptoms after therapy. We continue this GA in an even larger prospective multicenter cohort to determine each test's predictive power for survival and treatment toxicity.

Session topic: E-poster
Keyword(s): Comorbidities, Elderly, Multiple myeloma
{{ help_message }}
{{filter}}


