A POOLED ANALYSIS OF THE IMPACT OF AGE ON OUTCOMES IN PATIENTS WITH REFRACTORY OR RELAPSED AND REFRACTORY MULTIPLE MYELOMA TREATED WITH POMALIDOMIDE + LOW-DOSE DEXAMETHASONE
(Abstract release date: 05/19/16)
EHA Library. Palumbo A. 06/09/16; 132844; E1295

Prof. Antonio Palumbo
Contributions
Contributions
Abstract
Abstract: E1295
Type: Eposter Presentation
Background
The survival duration of patients with multiple myeloma is inversely proportional to their age (Pulte et al, Oncologist, 2011). Pomalidomide (POM), a distinct immunomodulatory agent with tumoricidal and immunoregulatory effects, plus low-dose dexamethasone (LoDEX) is approved in the United States and European Union for the treatment of patients with relapsed and refractory multiple myeloma (RRMM) who have had ≥ 2 prior therapies, including lenalidomide and a proteasome inhibitor (bortezomib in the European Union). Approval was based on 2 phase 3 studies (Richardson, Blood, 2014; San Miguel, Lancet Oncol, 2013), and the regimen has been further evaluated in a large confirmatory study (Dimopoulos ASH 2015).
Aims
To conduct a pooled analysis of the impact of age on outcomes in patients treated with POM + LoDEX in 3 large trials.
Methods
Patients who provided informed consent, had ≥ 2 prior therapies, including lenalidomide and bortezomib, and progressed on or within 60 days of their last therapy were enrolled in the 3 trials. Patients received POM 4 mg/day on days 1‐21 of each 28-day cycle and LoDEX 40 mg (20 mg for those > 75 years of age) weekly until disease progression or unacceptable toxicity. Thromboprophylaxis was required. Outcomes analysis was conducted based on patient age subgroup (≤ 65, > 65, ≤ 75, and > 75 years).
Results
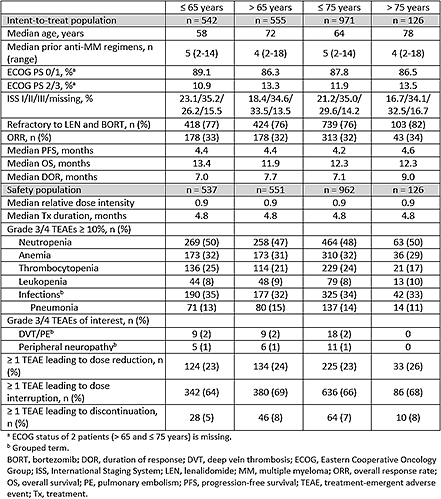
In total, 1097 patients were assigned to receive POM + LoDEX in the 3 trials and were included in the intent-to-treat population. Baseline demographic and disease characteristics were similar regardless of age (Table). Overall response rate (range, 32% - 34%) and median progression-free survival (range, 4.2-4.6 months) were also similar across the age groups. Median overall survival was slightly longer in the ≤ 65 vs > 65 subgroups (13.4 vs 11.9 months) but was similar in the ≤ 75 and > 75 subgroups (12.3 months). The median duration of response was slightly longer in the > 65 and > 75 subgroups (7.7 and 9.0 months, respectively) compared with the ≤ 65 and ≤ 75 subgroups (7.0 and 7.1 months, respectively). In the 1088 patients who received at least 1 dose of POM + LoDEX (safety population), the median relative dose intensity was 0.9, and the median treatment duration was 4.8 months in each age subgroup. Grade 3/4 treatment-emergent adverse events (TEAEs), including neutropenia (range, 47% - 50%), anemia (range, 29% - 32%), thrombocytopenia (range, 17% - 25%), and infections (range, 32% - 35%) were similar across the age groups. Additionally, similar rates of TEAEs leading to dose reduction (range, 23% - 26%) and interruption (range, 64% - 69%) were observed in the four subgroups. POM discontinuation due to TEAEs was infrequent (range, 5% - 8%).
Conclusion
POM + LoDEX demonstrated efficacy in patients with RRMM, with similar progression-free survival and response rates regardless of age; safety and efficacy in patients > 75 years was comparable to that in patients < 75 years. Similarly, the exposure and safety profile were similar across the age groups. These results support POM 4 mg as an effective starting dose and use of POM + LoDEX, regardless of age, in patients with RRMM.
Session topic: E-poster
Type: Eposter Presentation
Background
The survival duration of patients with multiple myeloma is inversely proportional to their age (Pulte et al, Oncologist, 2011). Pomalidomide (POM), a distinct immunomodulatory agent with tumoricidal and immunoregulatory effects, plus low-dose dexamethasone (LoDEX) is approved in the United States and European Union for the treatment of patients with relapsed and refractory multiple myeloma (RRMM) who have had ≥ 2 prior therapies, including lenalidomide and a proteasome inhibitor (bortezomib in the European Union). Approval was based on 2 phase 3 studies (Richardson, Blood, 2014; San Miguel, Lancet Oncol, 2013), and the regimen has been further evaluated in a large confirmatory study (Dimopoulos ASH 2015).
Aims
To conduct a pooled analysis of the impact of age on outcomes in patients treated with POM + LoDEX in 3 large trials.
Methods
Patients who provided informed consent, had ≥ 2 prior therapies, including lenalidomide and bortezomib, and progressed on or within 60 days of their last therapy were enrolled in the 3 trials. Patients received POM 4 mg/day on days 1‐21 of each 28-day cycle and LoDEX 40 mg (20 mg for those > 75 years of age) weekly until disease progression or unacceptable toxicity. Thromboprophylaxis was required. Outcomes analysis was conducted based on patient age subgroup (≤ 65, > 65, ≤ 75, and > 75 years).
Results
In total, 1097 patients were assigned to receive POM + LoDEX in the 3 trials and were included in the intent-to-treat population. Baseline demographic and disease characteristics were similar regardless of age (Table). Overall response rate (range, 32% - 34%) and median progression-free survival (range, 4.2-4.6 months) were also similar across the age groups. Median overall survival was slightly longer in the ≤ 65 vs > 65 subgroups (13.4 vs 11.9 months) but was similar in the ≤ 75 and > 75 subgroups (12.3 months). The median duration of response was slightly longer in the > 65 and > 75 subgroups (7.7 and 9.0 months, respectively) compared with the ≤ 65 and ≤ 75 subgroups (7.0 and 7.1 months, respectively). In the 1088 patients who received at least 1 dose of POM + LoDEX (safety population), the median relative dose intensity was 0.9, and the median treatment duration was 4.8 months in each age subgroup. Grade 3/4 treatment-emergent adverse events (TEAEs), including neutropenia (range, 47% - 50%), anemia (range, 29% - 32%), thrombocytopenia (range, 17% - 25%), and infections (range, 32% - 35%) were similar across the age groups. Additionally, similar rates of TEAEs leading to dose reduction (range, 23% - 26%) and interruption (range, 64% - 69%) were observed in the four subgroups. POM discontinuation due to TEAEs was infrequent (range, 5% - 8%).
Conclusion
POM + LoDEX demonstrated efficacy in patients with RRMM, with similar progression-free survival and response rates regardless of age; safety and efficacy in patients > 75 years was comparable to that in patients < 75 years. Similarly, the exposure and safety profile were similar across the age groups. These results support POM 4 mg as an effective starting dose and use of POM + LoDEX, regardless of age, in patients with RRMM.

Session topic: E-poster
Abstract: E1295
Type: Eposter Presentation
Background
The survival duration of patients with multiple myeloma is inversely proportional to their age (Pulte et al, Oncologist, 2011). Pomalidomide (POM), a distinct immunomodulatory agent with tumoricidal and immunoregulatory effects, plus low-dose dexamethasone (LoDEX) is approved in the United States and European Union for the treatment of patients with relapsed and refractory multiple myeloma (RRMM) who have had ≥ 2 prior therapies, including lenalidomide and a proteasome inhibitor (bortezomib in the European Union). Approval was based on 2 phase 3 studies (Richardson, Blood, 2014; San Miguel, Lancet Oncol, 2013), and the regimen has been further evaluated in a large confirmatory study (Dimopoulos ASH 2015).
Aims
To conduct a pooled analysis of the impact of age on outcomes in patients treated with POM + LoDEX in 3 large trials.
Methods
Patients who provided informed consent, had ≥ 2 prior therapies, including lenalidomide and bortezomib, and progressed on or within 60 days of their last therapy were enrolled in the 3 trials. Patients received POM 4 mg/day on days 1‐21 of each 28-day cycle and LoDEX 40 mg (20 mg for those > 75 years of age) weekly until disease progression or unacceptable toxicity. Thromboprophylaxis was required. Outcomes analysis was conducted based on patient age subgroup (≤ 65, > 65, ≤ 75, and > 75 years).
Results
In total, 1097 patients were assigned to receive POM + LoDEX in the 3 trials and were included in the intent-to-treat population. Baseline demographic and disease characteristics were similar regardless of age (Table). Overall response rate (range, 32% - 34%) and median progression-free survival (range, 4.2-4.6 months) were also similar across the age groups. Median overall survival was slightly longer in the ≤ 65 vs > 65 subgroups (13.4 vs 11.9 months) but was similar in the ≤ 75 and > 75 subgroups (12.3 months). The median duration of response was slightly longer in the > 65 and > 75 subgroups (7.7 and 9.0 months, respectively) compared with the ≤ 65 and ≤ 75 subgroups (7.0 and 7.1 months, respectively). In the 1088 patients who received at least 1 dose of POM + LoDEX (safety population), the median relative dose intensity was 0.9, and the median treatment duration was 4.8 months in each age subgroup. Grade 3/4 treatment-emergent adverse events (TEAEs), including neutropenia (range, 47% - 50%), anemia (range, 29% - 32%), thrombocytopenia (range, 17% - 25%), and infections (range, 32% - 35%) were similar across the age groups. Additionally, similar rates of TEAEs leading to dose reduction (range, 23% - 26%) and interruption (range, 64% - 69%) were observed in the four subgroups. POM discontinuation due to TEAEs was infrequent (range, 5% - 8%).
Conclusion
POM + LoDEX demonstrated efficacy in patients with RRMM, with similar progression-free survival and response rates regardless of age; safety and efficacy in patients > 75 years was comparable to that in patients < 75 years. Similarly, the exposure and safety profile were similar across the age groups. These results support POM 4 mg as an effective starting dose and use of POM + LoDEX, regardless of age, in patients with RRMM.

Session topic: E-poster
Type: Eposter Presentation
Background
The survival duration of patients with multiple myeloma is inversely proportional to their age (Pulte et al, Oncologist, 2011). Pomalidomide (POM), a distinct immunomodulatory agent with tumoricidal and immunoregulatory effects, plus low-dose dexamethasone (LoDEX) is approved in the United States and European Union for the treatment of patients with relapsed and refractory multiple myeloma (RRMM) who have had ≥ 2 prior therapies, including lenalidomide and a proteasome inhibitor (bortezomib in the European Union). Approval was based on 2 phase 3 studies (Richardson, Blood, 2014; San Miguel, Lancet Oncol, 2013), and the regimen has been further evaluated in a large confirmatory study (Dimopoulos ASH 2015).
Aims
To conduct a pooled analysis of the impact of age on outcomes in patients treated with POM + LoDEX in 3 large trials.
Methods
Patients who provided informed consent, had ≥ 2 prior therapies, including lenalidomide and bortezomib, and progressed on or within 60 days of their last therapy were enrolled in the 3 trials. Patients received POM 4 mg/day on days 1‐21 of each 28-day cycle and LoDEX 40 mg (20 mg for those > 75 years of age) weekly until disease progression or unacceptable toxicity. Thromboprophylaxis was required. Outcomes analysis was conducted based on patient age subgroup (≤ 65, > 65, ≤ 75, and > 75 years).
Results
In total, 1097 patients were assigned to receive POM + LoDEX in the 3 trials and were included in the intent-to-treat population. Baseline demographic and disease characteristics were similar regardless of age (Table). Overall response rate (range, 32% - 34%) and median progression-free survival (range, 4.2-4.6 months) were also similar across the age groups. Median overall survival was slightly longer in the ≤ 65 vs > 65 subgroups (13.4 vs 11.9 months) but was similar in the ≤ 75 and > 75 subgroups (12.3 months). The median duration of response was slightly longer in the > 65 and > 75 subgroups (7.7 and 9.0 months, respectively) compared with the ≤ 65 and ≤ 75 subgroups (7.0 and 7.1 months, respectively). In the 1088 patients who received at least 1 dose of POM + LoDEX (safety population), the median relative dose intensity was 0.9, and the median treatment duration was 4.8 months in each age subgroup. Grade 3/4 treatment-emergent adverse events (TEAEs), including neutropenia (range, 47% - 50%), anemia (range, 29% - 32%), thrombocytopenia (range, 17% - 25%), and infections (range, 32% - 35%) were similar across the age groups. Additionally, similar rates of TEAEs leading to dose reduction (range, 23% - 26%) and interruption (range, 64% - 69%) were observed in the four subgroups. POM discontinuation due to TEAEs was infrequent (range, 5% - 8%).
Conclusion
POM + LoDEX demonstrated efficacy in patients with RRMM, with similar progression-free survival and response rates regardless of age; safety and efficacy in patients > 75 years was comparable to that in patients < 75 years. Similarly, the exposure and safety profile were similar across the age groups. These results support POM 4 mg as an effective starting dose and use of POM + LoDEX, regardless of age, in patients with RRMM.

Session topic: E-poster
{{ help_message }}
{{filter}}


