EFFICACY AND SAFETY OF ORAL IXAZOMIB-LENALIDOMIDE-DEXAMETHASONE (IRD) VS PLACEBO-RD IN RELAPSED/REFRACTORY MULTIPLE MYELOMA PATIENTS: IMPACT OF PRIOR THERAPY IN THE PHASE 3 TOURMALINE-MM1 STUDY
(Abstract release date: 05/19/16)
EHA Library. Victoria Mateos M. 06/09/16; 132825; E1276

Maria Victoria Mateos
Contributions
Contributions
Abstract
Abstract: E1276
Type: Eposter Presentation
Background
The TOURMALINE-MM1 study (NCT01564537) demonstrated improved progression-free survival (PFS) with the all-oral combination of IRd vs placebo-Rd (median 20.6 vs 14.7 months; HR 0.74) in patients (pts) with relapsed/refractory multiple myeloma (RRMM)(Moreau et al, ASH 2015). Based on this study ixazomib was approved by the US FDA in combination with Rd for the treatment of pts with MM who have received at least one prior therapy.
Aims
To analyze the efficacy and safety of IRd vs placebo-Rd according to prior proteasome inhibitor (PI) and prior thalidomide (thal)/lenalidomide (R) exposure.
Methods
Pts with RRMM were randomized 1:1 to receive IRd or placebo-Rd (ixazomib 4 mg or matching placebo on days 1, 8, and 15, plus lenalidomide 25 mg on days 1–21 and dexamethasone 40 mg on days 1, 8, 15, and 22, in 28-day cycles) until disease progression or unacceptable toxicity. Pts were stratified by number of prior therapies (1 vs 2 or 3), PI-exposed vs PI-naïve status, and International Staging System (ISS) stage I or II vs III. Pts who had received prior therapy with PI- and thal/R-based regimens, and pts who were refractory to thal, were eligible for inclusion. However, pts who were refractory to PI- or R-based prior therapy were not included.
Results
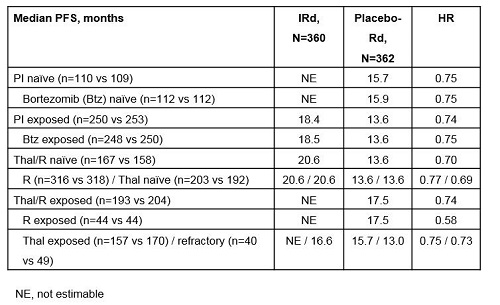
Of 722 pts, 69% had prior PI therapy (<1% prior carfilzomib) and 55% had prior thal/R therapy, including 45% prior thal (12% thal-refractory) and 12% prior R. Prior therapies were balanced between arms. At the primary analysis (median follow-up ~15 months), consistent PFS benefit was seen with IRd regardless of prior PI or thal/R exposure (Table). Overall response rate (ORR) with IRd vs placebo-Rd appeared generally similar across subgroups (PI-naïve: 81% vs 74%; PI-exposed: 77% vs 70%; thal/R-naïve: 80% vs 77%; R-naïve: 78% vs 73%) but slightly lower in thal-refractory pts (70% vs 57%), while ORR in the placebo-Rd arm appeared somewhat lower in thal/R-exposed (77% vs 67%) and R-exposed pts (77% vs 59%). Complete response plus very good partial response (CR+VGPR) rates with IRd vs placebo-Rd appeared generally similar in PI-naïve (54% vs 37%), PI-exposed (46% vs 40%), thal/R-naïve (51% vs 44%), thal/R-exposed (45% vs 35%), R-naïve (48% vs 39%), and R-exposed (45% vs 36%) pts, but lower in thal-refractory (30% vs 27%) pts. At a 23-month analysis, IRd safety profile was generally consistent regardless of prior PI or thal/R exposure. Rates of grade ≥3 AEs with IRd vs placebo-Rd were 76% vs 66% in PI-naïve, 73% vs 70% in PI-exposed, 75% vs 71% in thal/R-naïve, and 73% vs 67% in thal/R-exposed pts. Rates of individual AEs (all-grade and grade ≥3) with IRd vs placebo-Rd were similar and consistent with those reported for the overall patient population regardless of prior PI or thal/R exposure, including common AEs such as GI and hematologic toxicities, rash, and neuropathy, with the exception of neutropenia in the PI-naïve population; overall rates were 29% vs 20%, including 21% vs 15% grade ≥3, whereas IRd and placebo-Rd rates were similar in the overall population and other subgroups. Rates of serious AEs with IRd vs placebo-Rd were 47% vs 40% in PI-naïve, 46% vs 53% in PI-exposed, 48% vs 51% in thal/R-naïve, and 45% vs 48% in thal/R-exposed pts.
Conclusion
The benefit of IRd vs placebo-Rd appeared consistent across subgroups defined by prior PI and thal/R exposure; IRd safety profile was also broadly consistent across subgroups.
Session topic: E-poster
Keyword(s): Multiple myeloma, Phase III, Proteasome inhibitor
Type: Eposter Presentation
Background
The TOURMALINE-MM1 study (NCT01564537) demonstrated improved progression-free survival (PFS) with the all-oral combination of IRd vs placebo-Rd (median 20.6 vs 14.7 months; HR 0.74) in patients (pts) with relapsed/refractory multiple myeloma (RRMM)(Moreau et al, ASH 2015). Based on this study ixazomib was approved by the US FDA in combination with Rd for the treatment of pts with MM who have received at least one prior therapy.
Aims
To analyze the efficacy and safety of IRd vs placebo-Rd according to prior proteasome inhibitor (PI) and prior thalidomide (thal)/lenalidomide (R) exposure.
Methods
Pts with RRMM were randomized 1:1 to receive IRd or placebo-Rd (ixazomib 4 mg or matching placebo on days 1, 8, and 15, plus lenalidomide 25 mg on days 1–21 and dexamethasone 40 mg on days 1, 8, 15, and 22, in 28-day cycles) until disease progression or unacceptable toxicity. Pts were stratified by number of prior therapies (1 vs 2 or 3), PI-exposed vs PI-naïve status, and International Staging System (ISS) stage I or II vs III. Pts who had received prior therapy with PI- and thal/R-based regimens, and pts who were refractory to thal, were eligible for inclusion. However, pts who were refractory to PI- or R-based prior therapy were not included.
Results
Of 722 pts, 69% had prior PI therapy (<1% prior carfilzomib) and 55% had prior thal/R therapy, including 45% prior thal (12% thal-refractory) and 12% prior R. Prior therapies were balanced between arms. At the primary analysis (median follow-up ~15 months), consistent PFS benefit was seen with IRd regardless of prior PI or thal/R exposure (Table). Overall response rate (ORR) with IRd vs placebo-Rd appeared generally similar across subgroups (PI-naïve: 81% vs 74%; PI-exposed: 77% vs 70%; thal/R-naïve: 80% vs 77%; R-naïve: 78% vs 73%) but slightly lower in thal-refractory pts (70% vs 57%), while ORR in the placebo-Rd arm appeared somewhat lower in thal/R-exposed (77% vs 67%) and R-exposed pts (77% vs 59%). Complete response plus very good partial response (CR+VGPR) rates with IRd vs placebo-Rd appeared generally similar in PI-naïve (54% vs 37%), PI-exposed (46% vs 40%), thal/R-naïve (51% vs 44%), thal/R-exposed (45% vs 35%), R-naïve (48% vs 39%), and R-exposed (45% vs 36%) pts, but lower in thal-refractory (30% vs 27%) pts. At a 23-month analysis, IRd safety profile was generally consistent regardless of prior PI or thal/R exposure. Rates of grade ≥3 AEs with IRd vs placebo-Rd were 76% vs 66% in PI-naïve, 73% vs 70% in PI-exposed, 75% vs 71% in thal/R-naïve, and 73% vs 67% in thal/R-exposed pts. Rates of individual AEs (all-grade and grade ≥3) with IRd vs placebo-Rd were similar and consistent with those reported for the overall patient population regardless of prior PI or thal/R exposure, including common AEs such as GI and hematologic toxicities, rash, and neuropathy, with the exception of neutropenia in the PI-naïve population; overall rates were 29% vs 20%, including 21% vs 15% grade ≥3, whereas IRd and placebo-Rd rates were similar in the overall population and other subgroups. Rates of serious AEs with IRd vs placebo-Rd were 47% vs 40% in PI-naïve, 46% vs 53% in PI-exposed, 48% vs 51% in thal/R-naïve, and 45% vs 48% in thal/R-exposed pts.
Conclusion
The benefit of IRd vs placebo-Rd appeared consistent across subgroups defined by prior PI and thal/R exposure; IRd safety profile was also broadly consistent across subgroups.

Session topic: E-poster
Keyword(s): Multiple myeloma, Phase III, Proteasome inhibitor
Abstract: E1276
Type: Eposter Presentation
Background
The TOURMALINE-MM1 study (NCT01564537) demonstrated improved progression-free survival (PFS) with the all-oral combination of IRd vs placebo-Rd (median 20.6 vs 14.7 months; HR 0.74) in patients (pts) with relapsed/refractory multiple myeloma (RRMM)(Moreau et al, ASH 2015). Based on this study ixazomib was approved by the US FDA in combination with Rd for the treatment of pts with MM who have received at least one prior therapy.
Aims
To analyze the efficacy and safety of IRd vs placebo-Rd according to prior proteasome inhibitor (PI) and prior thalidomide (thal)/lenalidomide (R) exposure.
Methods
Pts with RRMM were randomized 1:1 to receive IRd or placebo-Rd (ixazomib 4 mg or matching placebo on days 1, 8, and 15, plus lenalidomide 25 mg on days 1–21 and dexamethasone 40 mg on days 1, 8, 15, and 22, in 28-day cycles) until disease progression or unacceptable toxicity. Pts were stratified by number of prior therapies (1 vs 2 or 3), PI-exposed vs PI-naïve status, and International Staging System (ISS) stage I or II vs III. Pts who had received prior therapy with PI- and thal/R-based regimens, and pts who were refractory to thal, were eligible for inclusion. However, pts who were refractory to PI- or R-based prior therapy were not included.
Results
Of 722 pts, 69% had prior PI therapy (<1% prior carfilzomib) and 55% had prior thal/R therapy, including 45% prior thal (12% thal-refractory) and 12% prior R. Prior therapies were balanced between arms. At the primary analysis (median follow-up ~15 months), consistent PFS benefit was seen with IRd regardless of prior PI or thal/R exposure (Table). Overall response rate (ORR) with IRd vs placebo-Rd appeared generally similar across subgroups (PI-naïve: 81% vs 74%; PI-exposed: 77% vs 70%; thal/R-naïve: 80% vs 77%; R-naïve: 78% vs 73%) but slightly lower in thal-refractory pts (70% vs 57%), while ORR in the placebo-Rd arm appeared somewhat lower in thal/R-exposed (77% vs 67%) and R-exposed pts (77% vs 59%). Complete response plus very good partial response (CR+VGPR) rates with IRd vs placebo-Rd appeared generally similar in PI-naïve (54% vs 37%), PI-exposed (46% vs 40%), thal/R-naïve (51% vs 44%), thal/R-exposed (45% vs 35%), R-naïve (48% vs 39%), and R-exposed (45% vs 36%) pts, but lower in thal-refractory (30% vs 27%) pts. At a 23-month analysis, IRd safety profile was generally consistent regardless of prior PI or thal/R exposure. Rates of grade ≥3 AEs with IRd vs placebo-Rd were 76% vs 66% in PI-naïve, 73% vs 70% in PI-exposed, 75% vs 71% in thal/R-naïve, and 73% vs 67% in thal/R-exposed pts. Rates of individual AEs (all-grade and grade ≥3) with IRd vs placebo-Rd were similar and consistent with those reported for the overall patient population regardless of prior PI or thal/R exposure, including common AEs such as GI and hematologic toxicities, rash, and neuropathy, with the exception of neutropenia in the PI-naïve population; overall rates were 29% vs 20%, including 21% vs 15% grade ≥3, whereas IRd and placebo-Rd rates were similar in the overall population and other subgroups. Rates of serious AEs with IRd vs placebo-Rd were 47% vs 40% in PI-naïve, 46% vs 53% in PI-exposed, 48% vs 51% in thal/R-naïve, and 45% vs 48% in thal/R-exposed pts.
Conclusion
The benefit of IRd vs placebo-Rd appeared consistent across subgroups defined by prior PI and thal/R exposure; IRd safety profile was also broadly consistent across subgroups.

Session topic: E-poster
Keyword(s): Multiple myeloma, Phase III, Proteasome inhibitor
Type: Eposter Presentation
Background
The TOURMALINE-MM1 study (NCT01564537) demonstrated improved progression-free survival (PFS) with the all-oral combination of IRd vs placebo-Rd (median 20.6 vs 14.7 months; HR 0.74) in patients (pts) with relapsed/refractory multiple myeloma (RRMM)(Moreau et al, ASH 2015). Based on this study ixazomib was approved by the US FDA in combination with Rd for the treatment of pts with MM who have received at least one prior therapy.
Aims
To analyze the efficacy and safety of IRd vs placebo-Rd according to prior proteasome inhibitor (PI) and prior thalidomide (thal)/lenalidomide (R) exposure.
Methods
Pts with RRMM were randomized 1:1 to receive IRd or placebo-Rd (ixazomib 4 mg or matching placebo on days 1, 8, and 15, plus lenalidomide 25 mg on days 1–21 and dexamethasone 40 mg on days 1, 8, 15, and 22, in 28-day cycles) until disease progression or unacceptable toxicity. Pts were stratified by number of prior therapies (1 vs 2 or 3), PI-exposed vs PI-naïve status, and International Staging System (ISS) stage I or II vs III. Pts who had received prior therapy with PI- and thal/R-based regimens, and pts who were refractory to thal, were eligible for inclusion. However, pts who were refractory to PI- or R-based prior therapy were not included.
Results
Of 722 pts, 69% had prior PI therapy (<1% prior carfilzomib) and 55% had prior thal/R therapy, including 45% prior thal (12% thal-refractory) and 12% prior R. Prior therapies were balanced between arms. At the primary analysis (median follow-up ~15 months), consistent PFS benefit was seen with IRd regardless of prior PI or thal/R exposure (Table). Overall response rate (ORR) with IRd vs placebo-Rd appeared generally similar across subgroups (PI-naïve: 81% vs 74%; PI-exposed: 77% vs 70%; thal/R-naïve: 80% vs 77%; R-naïve: 78% vs 73%) but slightly lower in thal-refractory pts (70% vs 57%), while ORR in the placebo-Rd arm appeared somewhat lower in thal/R-exposed (77% vs 67%) and R-exposed pts (77% vs 59%). Complete response plus very good partial response (CR+VGPR) rates with IRd vs placebo-Rd appeared generally similar in PI-naïve (54% vs 37%), PI-exposed (46% vs 40%), thal/R-naïve (51% vs 44%), thal/R-exposed (45% vs 35%), R-naïve (48% vs 39%), and R-exposed (45% vs 36%) pts, but lower in thal-refractory (30% vs 27%) pts. At a 23-month analysis, IRd safety profile was generally consistent regardless of prior PI or thal/R exposure. Rates of grade ≥3 AEs with IRd vs placebo-Rd were 76% vs 66% in PI-naïve, 73% vs 70% in PI-exposed, 75% vs 71% in thal/R-naïve, and 73% vs 67% in thal/R-exposed pts. Rates of individual AEs (all-grade and grade ≥3) with IRd vs placebo-Rd were similar and consistent with those reported for the overall patient population regardless of prior PI or thal/R exposure, including common AEs such as GI and hematologic toxicities, rash, and neuropathy, with the exception of neutropenia in the PI-naïve population; overall rates were 29% vs 20%, including 21% vs 15% grade ≥3, whereas IRd and placebo-Rd rates were similar in the overall population and other subgroups. Rates of serious AEs with IRd vs placebo-Rd were 47% vs 40% in PI-naïve, 46% vs 53% in PI-exposed, 48% vs 51% in thal/R-naïve, and 45% vs 48% in thal/R-exposed pts.
Conclusion
The benefit of IRd vs placebo-Rd appeared consistent across subgroups defined by prior PI and thal/R exposure; IRd safety profile was also broadly consistent across subgroups.

Session topic: E-poster
Keyword(s): Multiple myeloma, Phase III, Proteasome inhibitor
{{ help_message }}
{{filter}}


